Gold Silicates

The existence of auro-silicates is now admitted without dispute, and gold has for centuries been used to impart colour to glasses, the method used being as follows: A solution of chloride of gold is added to a mixture of sand with alkalies and alkaline earths or lead, and the whole is then fused, and colourless […]
Gold Sulfite

Sulphites of Gold, alkaline sulphites, or sulphur dioxide, which reduce gold trichloride easily, do not produce the same effect on a solution of an alkaline aurate. If sodium bisulphite is added to a boiling solution of sodium aurate (NaAuO2) a yellowish precipitate is formed, soluble in excess of sodium bisulphite, and consisting of a double […]
Gold Oxides
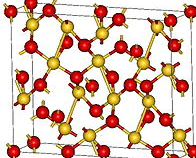
Aurous Oxide, Au2O This gold oxide is prepared by decomposing aurous chloride, AuCl, or the corresponding bromide by potash in the cold (Berzelius) when a violet precipitate forms, which is blackish when moist, but greyish when dry. When freshly precipitated it is soluble both in alkalies and in cold water, forming an indigo blue solution, with […]
Gold Cyanide Formulas

Cyanogen and gold unite in two proportions, forming aurous and auric cyanides, but the latter is only known with certainty in combination. Aurous Cyanide, AuCy, is obtained by heating aurocyanide of potassium, KAuCy2, with hydrochloric or nitric acid and washing with water. It is a lemon-yellow crystalline powder, insoluble in water, and unaltered by exposure […]
Gold Bromides

Gold Protobromide, AuBr, is a yellowish-green powder obtained by heating the tribromide to about 140°. It is insoluble in water, but is decomposed by it, metalic gold and the tribromide being formed; the change is especially rapid on boiling, and is hastened by the presence of hydrobromic acid. Auro-auric Bromide, Au2Br4, is produced by the […]
Gold Chloride
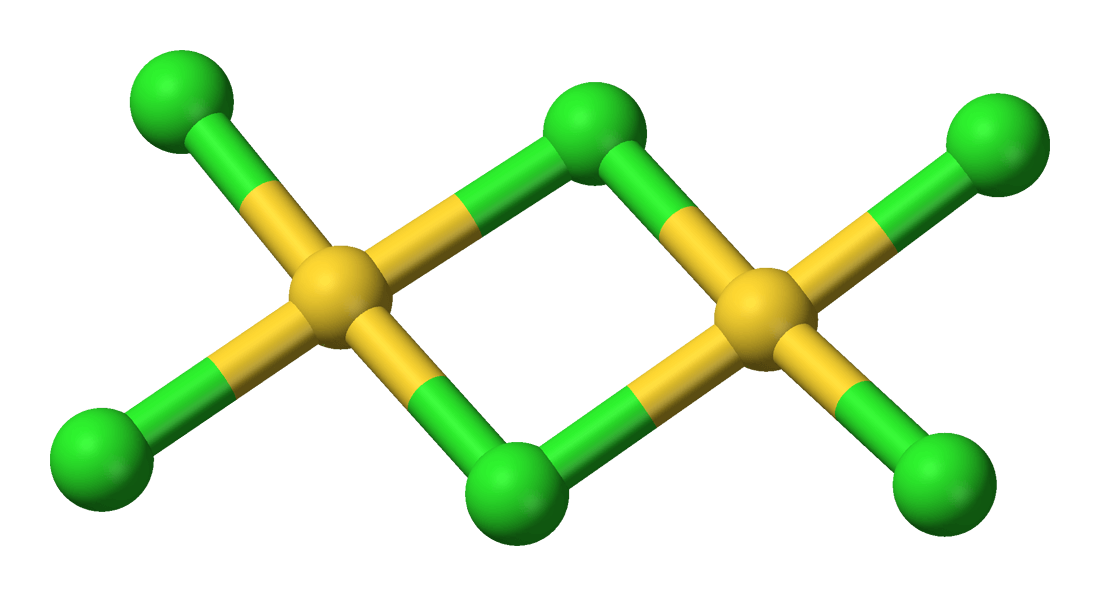
Gold Monochloride or Aurous Gold Chloride “AuCl” is a salt is prepared by heating the trichloride to 185° in air for twelve hours. It is non-volatile and unaltered at ordinary temperatures and pressure by dry air, even when exposed to light, but begins to decompose at temperatures above 160°, and the decomposition is complete if it is […]
Gold and Copper Alloys
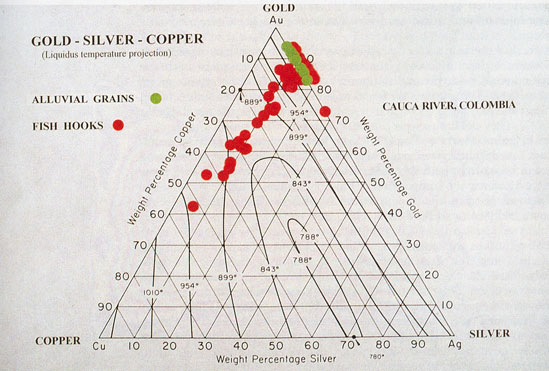
Gold and Copper Alloys dissolve in one another in all proportions, forming a complete series of homogeneous alloys, which are less malleable, harder, more elastic, and more fusible than gold, and possess a reddish tint. Those with less than 12 per cent, of copper are fairly malleable; when more than this is present they are […]
Gold and Silver Alloys
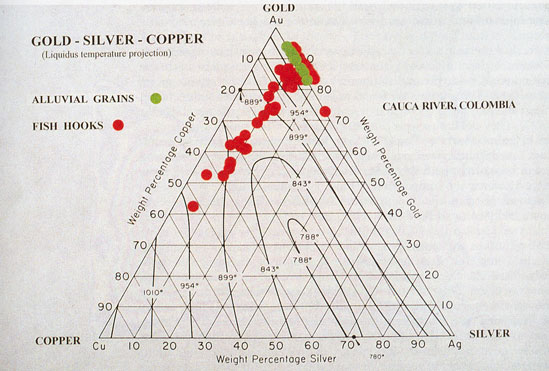
Gold and silver unite in all proportions, yielding alloys which are harder, more fusible, and more elastic than either metal. The hardest is that containing two parts of gold to one of silver. The colour of gold is sensibly lowered by the addition of very small quantities of silver, and on increasing the proportion of […]
Gold Alloys

Gold can alloy with almost all other metals, but most of the bodies thus formed are of little or no practical importance. Tin, zinc, arsenic and antimony unite with gold with contraction, and form pale yellow or grey coloured, hard, brittle and easily fusible alloys, of which all, except those containing zinc, are soluble with […]
Solubility of Gold
Gold is readily soluble in aqua regia, or in any other mixture producing nascent chlorine, among such mixtures being solutions of: nitrates, chlorides, and sulphates — e.g., bisulphate of soda, nitrate of soda, and common salt; chlorides and some sulphates—e.g., ferric sulphate; hydrochloric acid and potassium chlorate; bleaching powder and acids, or salts such as […]
