Method to Assay for Soluble Sulphides in Leach Solution
This is seldom called for, but it happens occasionally that an appreciable amount of sulphide is developed in the cyanidation of concentrate, and some sulphide is sometimes apparent in the press effluent from aluminium precipitation which it may be desirable to estimate. For such small amounts Clennell recommends the colorimetric method. For this purpose a […]
Determine Oxygen Content in Leach Solution

Here is a way how to determine the oxygen content during gold leaching and Estimation of available Oxygen Content in Cyanide Solutions. Until recently no simple and reliable method has been known for the estimation of free oxygen in working cyanide solutions so that the one here described supplies something that has long been needed. The matter […]
Assay Method of Gold or Silver in Leach Solution
Gold & Silver Assay Method No. 1 Evaporate a known quantity in a square boat made of lead foil, scorify, and cupel. This is not reliable for low grade solutions because the amount that can be taken for assay is too small. Gold & Silver Assay Method No. 2 Evaporate a known quantity in a large porcelain dish whose […]
Compare NaCN Sodium Cyanide VS KCN Potassium Cyanide

There is probably no potassium cyanide now made for commercial purposes. With the development of the cyanide process the custom grew up, for economic reasons, of manufacturing what was called a “double salt” composed of potassium and sodium cyanides in varying proportions. This material was compounded so as to have a cyanogen content of 98% […]
List Sources of Cyanide Loss
Below is a list of things/factor which can cause cyanide loses in the leach process: The Precious Metals The amount of cyanide that combines with the gold in an average ore is negligible, but with silver it is different owing to the much larger quantity of metal that has to be dealt with to produce […]
Gold Circuit Carbon Sampling for Inventory and Movement

Metallurgical performance of gold and silver mineral processing flowsheets is typically evaluated using assay balance and production balance techniques. With assay balance the mill feed, tailings, and some intermediate streams are sampled and assayed. Gold circuit performance can then be evaluated using mass balance techniques. Unsteady plant conditions and difficulties in sampling and measuring moving streams are some problems with this method. Production balance measures […]
Cyanide Reflux Distillation Apparatus
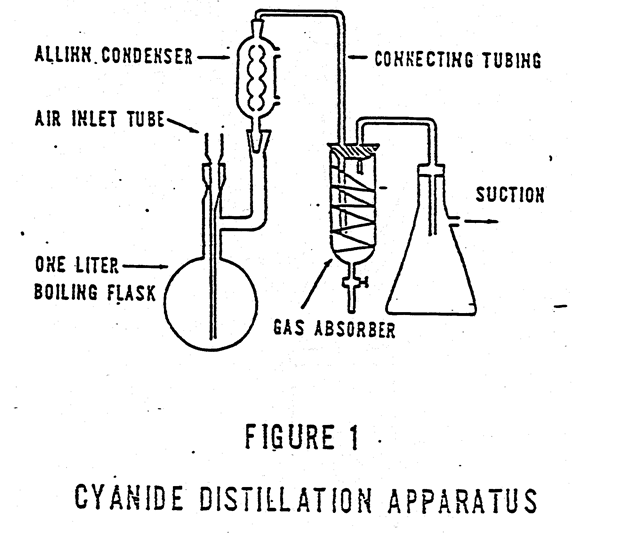
Distillation Procedure Samples without sulfide. Place 500 mL of the combined sample or an aliquot diluted to 500 mL in the 1 liter boiling flask. Pipet 50 mL of 1.25 N sodium hydroxide into the absorbing tube. If the apparatus in Figure 1 is used, add distilled water until the spiral is covered. Connect the […]
Assay gold and silver in cyanide solutions
In the assay of gold and silver in cyanide solutions the degree of accuracy and the speed desired are the governing factors in the choice of methods used and the quantity of solution taken for the determination. Evaporation {Litharge) Method To an evaporating dish add about 50 grams litharge and 146 to 292 cc cyanide solution. […]
Assaying for Zinc in Cyanide Solution
Zinc usually occurs in cyanide solutions as the double cyanide, but under certain conditions, e.g., in dilute solutions, a portion of the zinc may be present as zinc cyanide. It is possible that some may also exist as an alkaline zincate. Procedure To 500 cc of solution add 10 cc HCl, 10 cc HNO3, and […]
Test for Traces of Cyanide
Here is a Qualitative Test Method to look for Cyanide Traces. To 500 to 1000 cc of the solution to be tested add 1 to 2 cc ammonium sulphide, (NH4)2S, and evaporate just to dryness. The final stages of evaporation should be done slowly. Cool, add 10 cc water, stir well, let settle, and filter. To the filtrate […]
