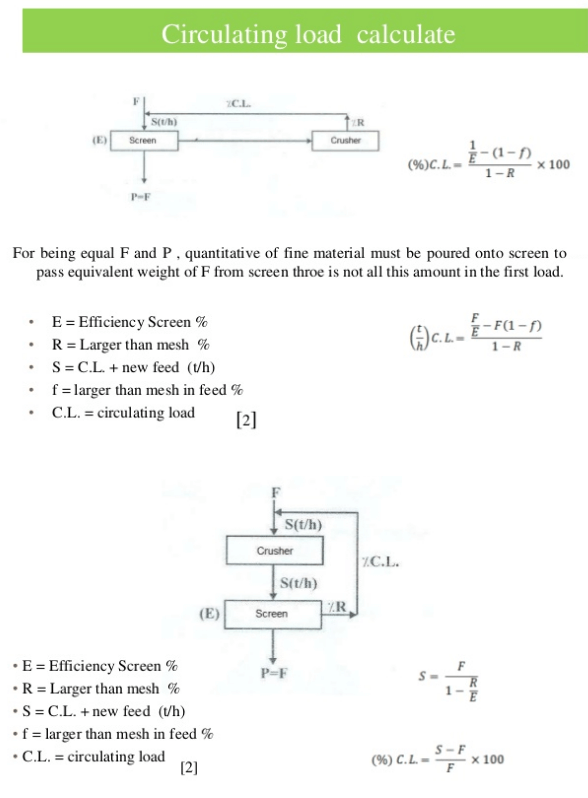
Circulating Load in a Crushing Circuit
Assaying for Cyanide
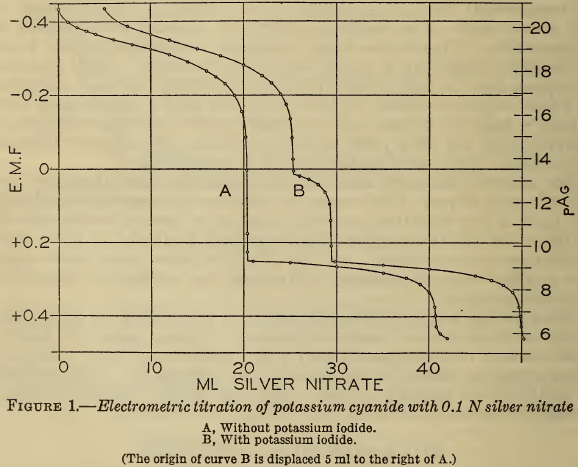
Methods for the analysis of cyanide silver-plating solutions were studied, including the determination of free cyanide, total cyanide, carbonate, chloride, ammonia, silver, iron, copper, and mercury. Electrometric titrations showed that the Liebig method for alkali cyanide is correct to better than 0.2 per cent. Addition of iodide makes the method still more accurate and overcomes […]
FREE Cyanide Assay Method
A simple example of the application of a complexation reaction to a titration procedure is the titration of cyanides with silver nitrate solution. When a solution of silver nitrate is added to a solution containing cyanide ions (e.g. an alkali cyanide) a white precipitate is formed when the two liquids first come into contact with […]
How to Collect a Sample for Cyanide Analysis
It is important to make use of composite sampling and to take at least five samples. Grab samples may be representative of flow during a short period and any other sample shown to be representative of waters being sampled may be applicable. The collection of samples for cyanide determination will require treatment to preserve the […]
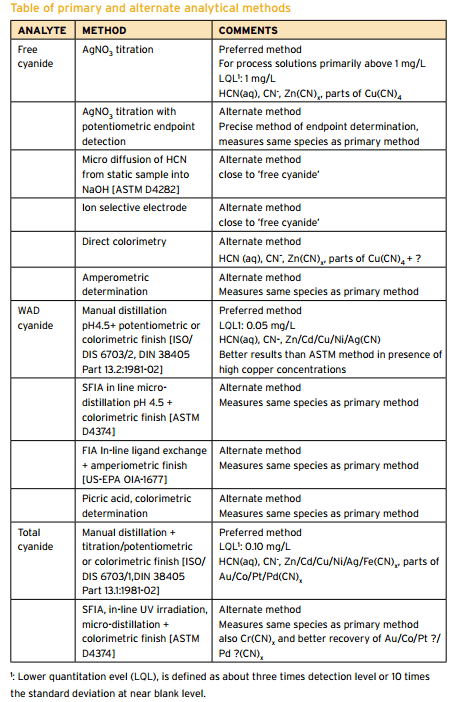
Cyanide Assay Methods
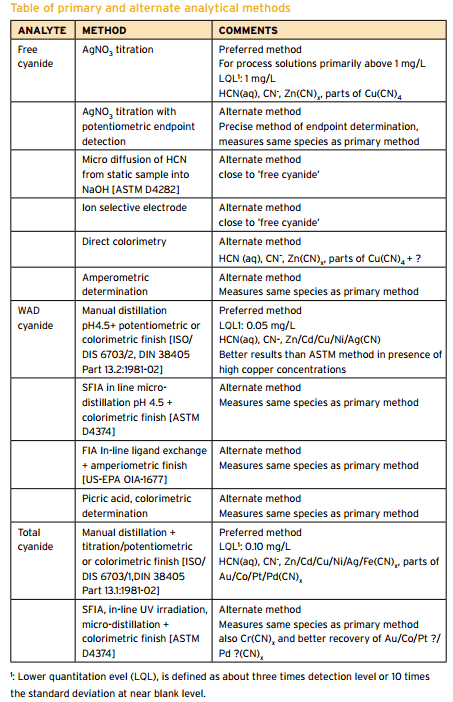
Summary of CN Analysis Methods Description Name Method Description Potential Interferences Measurement Free Cyanide ASTM D 4282 Passive Diffusion at pH 6 and room temperature • Storage • Measurement • Manual chlorination of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid. • Maximum absorbance is determined by manual colorimetry. ASTM D 7237 Flow Injection […]
Cyanide in Nature
Carbon and nitrogen, the two elements that make up cyanide, are present all around us. Together they make up almost 80% of the air we breathe, and both are present in the organic molecules that are the basis of all life forms. Hydrogen cyanide was formed in the earliest stages of the development of our planet as a […]
Hydrogen Peroxide Cyanide Destruction Plant
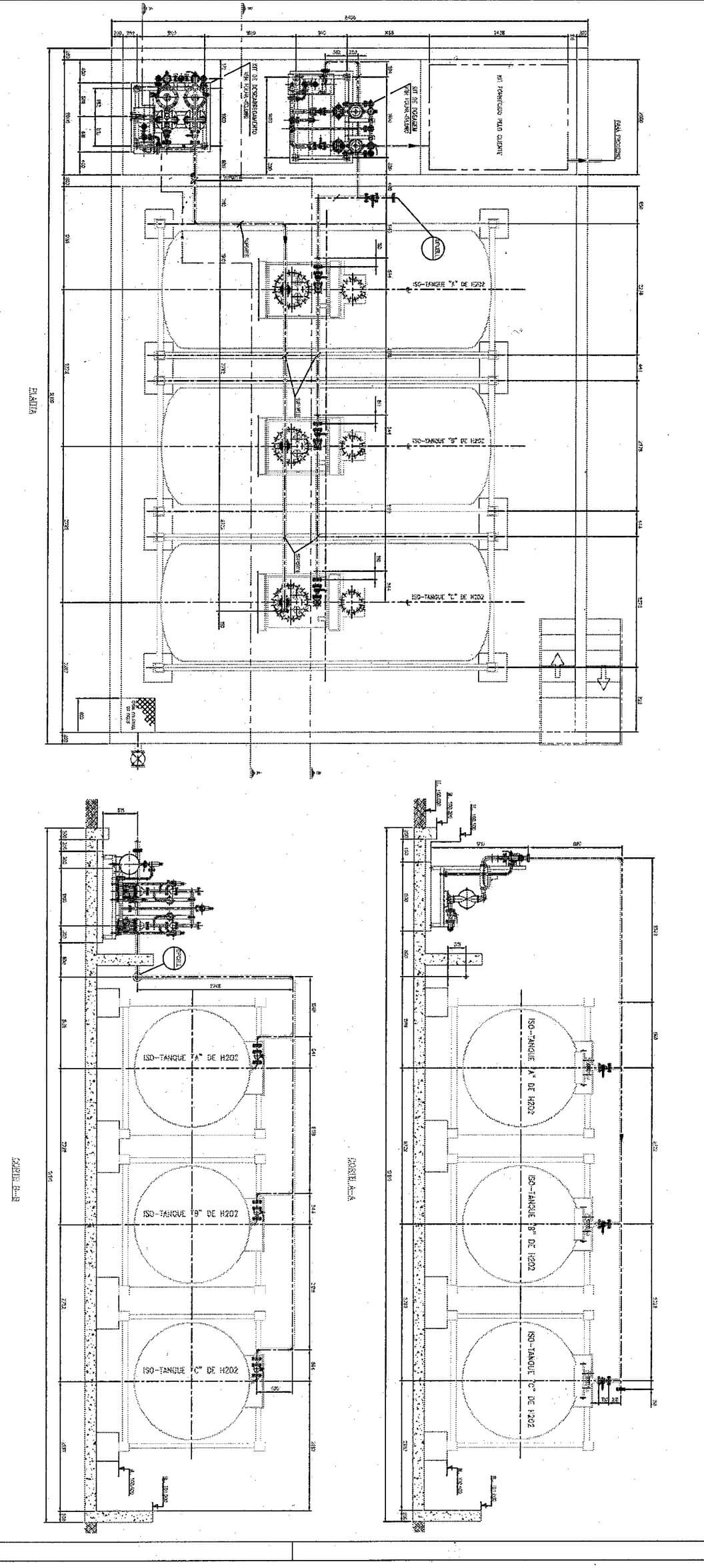
Hydrogen peroxide (H2O2) oxidizes free and weakly-complexed cyanide in a one-step reaction to yield cyanate. Metals such as copper are precipitated simultaneously as hydroxides. CN- + H2O2 → OCN- + H2O………………………………………………………….(1) 2 Cu(CN)3²- + 7H2O2 + 2OH- → 6OCN- + 2Cu(OH)2 + 6H2O…………………………………..(2) The strongest metal-cyanide complexes commonly encountered in the effluents from gold and […]
Equipment Sizing: Crusher or Grinding Mill
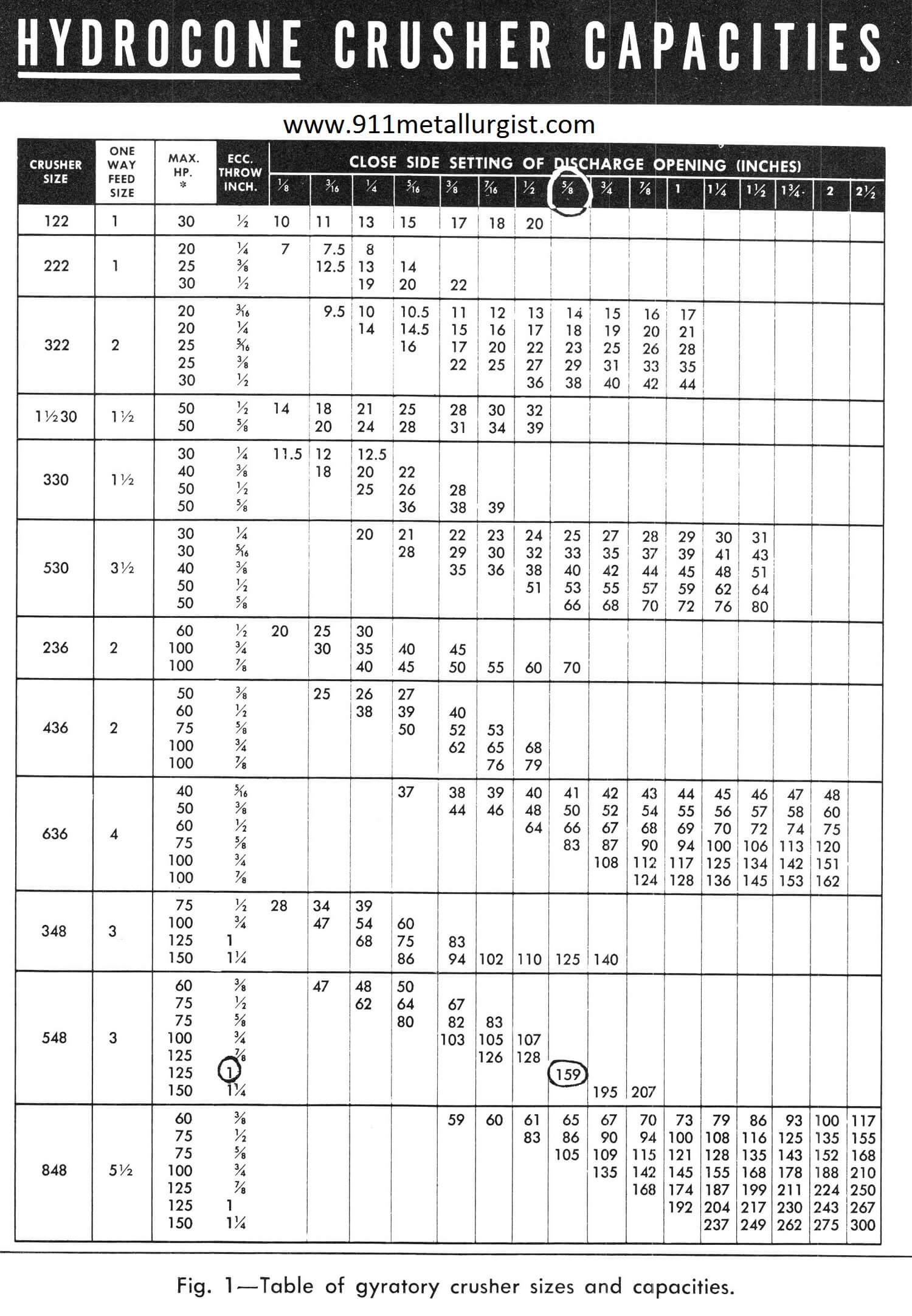
UNTIL THE THIRD THEORY OF COMMINUTION of “Work Index” method of determining crushing and grinding mill size was introduced, there was no way of accurately figuring the most applicable, most economical size of crushing and grinding mill. Naturally, with little or no factual operating data correlated in useful form, it was easy enough, even for […]
7 Rare Earth Elements that Run our World {Infographic}

SOURCES Alonso, E et al. 2012. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environmental Science & Technology. 46 (3406−3414) PubMed.gov Humphries, M. 2013 Rare Earth Elements: The Global Supply Chain. Congressional Research Service fas.org King, H. Rare Earth Elements and their Uses. geology.com Mineral Commodity Summaries. 2016. United States […]
Cyanide Destruction Methods and Processes

Detoxification processes are used to reduce the concentrations of toxic constituents in tailings streams and process solutions, either by dilution, removal, or conversion to a less toxic chemical form (sometimes referred to as“destruction” or “degradation” in the case of toxic cyanide species). The objective is to produce an effluent that meets limits or guidelines that have been set […]
