Laboratory Burette Supports and Holders

BURETTE SUPPORT—For one burette. Of wood, with adjustable cork lined clamp. BURETTE SUPPORT—For two burettes. Of wood, with adjustable cork lined clamps. SUPPORT, Burette, Castaloy, Double—One-piece, thick, molded opal-white glass base, with reinforcing ribs underneath, and rubber feet, with nickel plated support rod and Castaloy holder for supporting two burettes firmly and vertically. The opal-white of […]
Geology Videos
Free Cyanide Titration: Pyrrhotite & Sodium Sulphide, Thiosulphate, Thiocyanate, Ferrocyanide

When cyaniding a precious metal ore containing pyrrhotite, various compounds such as, thiosulphate, thiocyanate, ferrocyanide and soluble sulphide are formed and found in the cyanide solution. Soluble sulphides, if present at all, are found only in relatively small amounts; on the other hand thiosulphate, thiocyanate and ferrocyanide have been found in solutions in amounts of […]
GOLDIX
Discuss Goldix 1, 2 and 3
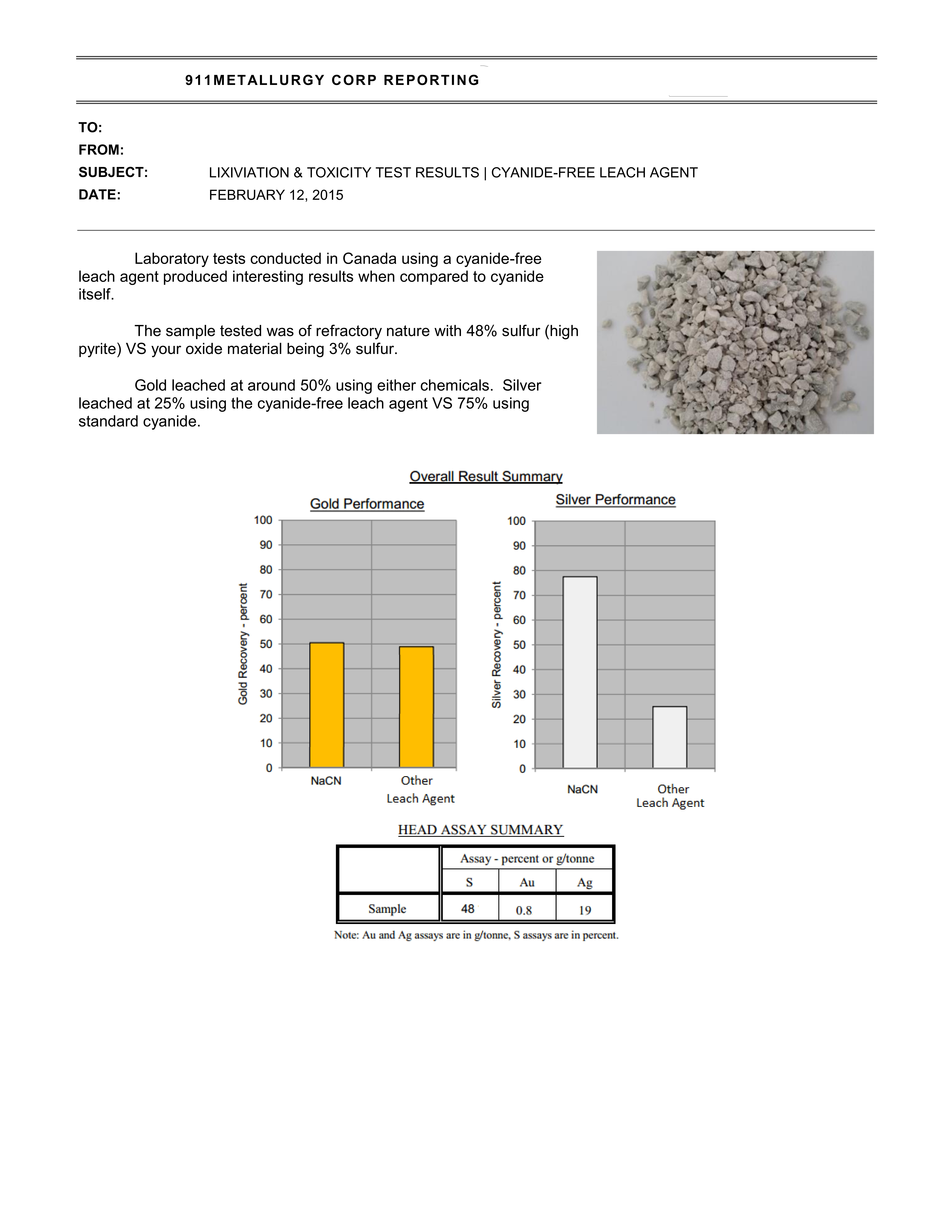
Metallurgist Handbook – Reference Tables & Charts
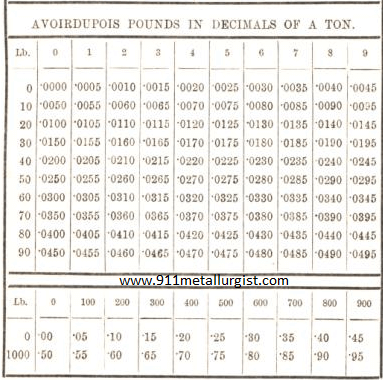
Metallurgist Handbook – Reference Tables & Charts International atomic weights Absolute Viscosity of water Thermometric Conversions Thermometric Conversions Fahrenheit to Centrigrade Absorption of Gases By Water Table of Pressure Boiling Point of Water at Various Pressures Stages in Decomposition of Pyrites Value of Lime as an Electrolyte Solubility of Lime in Water at Different Temperatures […]
Gold/Silver Leaching with Sodium Zinc Cyanide

Dissolution of Precious Metals in Sodium Zinc Cyanide: The dissolving effect of sodium zinc cyanide on precious metals has been the subject of much discussion. According to one researcher, pure K2Zn(CN)4 in the presence of oxygen dissolves gold with the formation of gold potassium cyanide and zinc oxide. Another researcher compared the dissolving effects of KCN and […]
Titration of Cyanide Solutions

Titration of Cyanide Solutions Containing Dissolved Zinc: Sodium zinc cyanide reacts with silver nitrate to precipitate zinc cyanide: Na2Zn(CN)4 + AgNO3 = Zn(CN)2 + NaAg(CN)2 + NaNO3 In the presence of an alkali, considerably more silver nitrate must be added to sodium zinc cyanide before a precipitate is formed. Many investigators have claimed that this […]
Zinc Cyanide & Silver Gold Precipitation
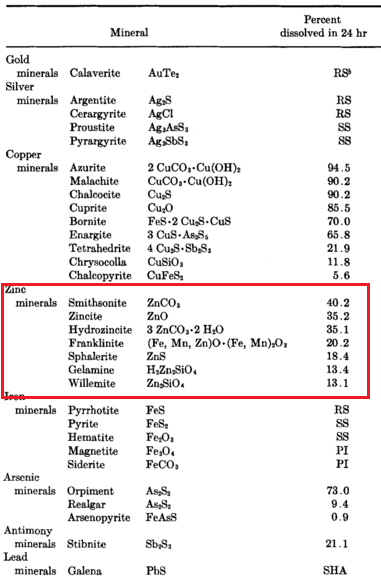
In cyanidation, zinc (the Merrill-Crowe Process) is practically the universal precipitant for precious metals dissolved in cyanide solutions although for specific reasons aluminium and charcoal have been used. During the reactions involved in precipitation, the greater part of the zinc dissolves in the cyanide solution and forms various cyanogen complexes such as sodium or calcium […]
Effect of Temperature on Gold Cyanidation

When heat is applied to a cyanide solution containing metallic gold, two opposing factors affect the rate of dissolution. The increase in temperature would be expected to increase the activity of the solution and thus increase the rate of dissolution of gold. At the same time the amount of oxygen in the solution would decrease […]
Effect of pH Alkalinity on Gold Leaching

The role pH has in affecting gold leaching rates by cyanide and the functions of calcium hydroxide in cyanidation are as follow: 1. For safety and to prevent loss of cyanide by hydrolysis. 2. To prevent loss of cyanide by the action of carbon dioxide in the air. 3. To decompose bicarbonates in mill water […]
