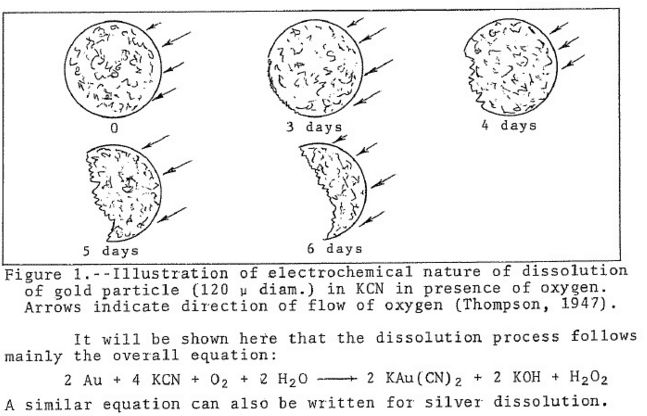
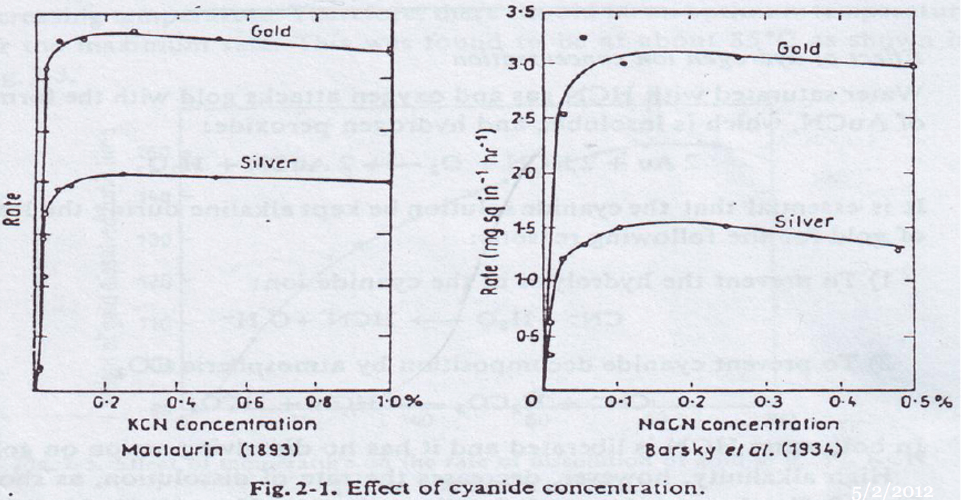
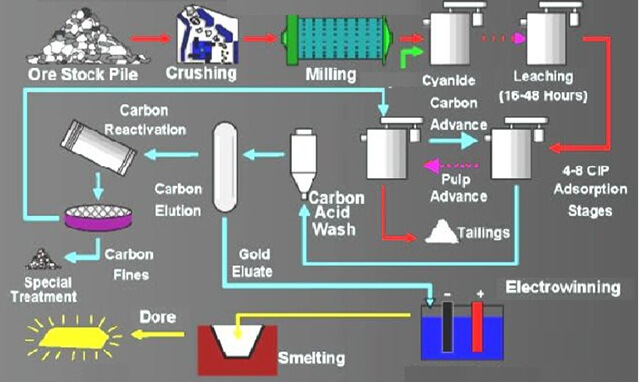
Titration of Cyanide Solutions
Titration of Cyanide Solutions Containing Dissolved Zinc: Sodium zinc cyanide reacts with silver nitrate to precipitate zinc cyanide: Na2Zn(CN)4 + AgNO3 = Zn(CN)2 + NaAg(CN)2 + NaNO3 In the presence of an alkali, considerably more silver nitrate must be added to sodium zinc cyanide before a precipitate is formed. Many investigators have claimed that this … Read more