In cyanidation, zinc (the Merrill-Crowe Process) is practically the universal precipitant for precious metals dissolved in cyanide solutions although for specific reasons aluminium and charcoal have been used. During the reactions involved in precipitation, the greater part of the zinc dissolves in the cyanide solution and forms various cyanogen complexes such as sodium or calcium zinc cyanide, zinc cyanide, zinc thiocyanate, zinc ferrocyanide. Other compounds may be formed such as sodium zincate and calcium zincate.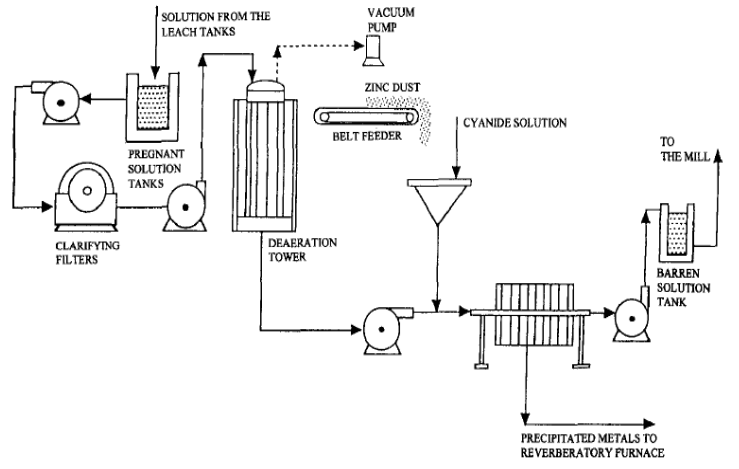
The amount of zinc used for the precipitation of precious metals depends to a large extent on the amount of precious metal in solution although other factors such as oxygen content, cyanide strength, and alkalinity play an important part. With gold ores, the pregnant solution going to precipitation rarely carries more than 0. 10 oz. of gold per ton and the amount of zinc required to precipitate this gold might be less than 0.5 oz. per ton. Consequently, such cyanide solutions are low in zinc content; the percentage present is rarely over 0.01 percent and frequently under 0.005 percent.
In cyaniding silver ores, however, the pregnant solution may carry up to 10 oz. of silver per ton. A correspondingly large amount of zinc is required to precipitate this silver with the result that solutions carrying up to 0.10 percent zinc are not unusual in silver cyanidation operations. Thus, any problems connected with the presence of zinc complexes in cyanidation are likely to be more apparent in silver operations than in gold.
Zinc Cyanogen Complexes and Zincates
The presence of zinc in cyanide solutions may be traced to sources other than the metallic zinc used for precipitation. Not infrequently, a precious metal ore contains zinc minerals such as sphalerite (ZnS), marmatite (ZnS plus FeS), smithsonite (ZnC03) and willemite (Zn2SiO4). All of these minerals will dissolve to a greater or lesser extent in cyanide solutions depending on the composition of the solutions and the characteristics of the minerals. The products of these reactions are various zinc cyanogen compounds and zincates, the properties of which are given below.
ZINC CYANIDE Zn(CN),: This compound is prepared by combining an alkaline cyanide solution and a soluble zinc salt in stoichiometric proportions:
ZnCL2 + 2 NaCN = Zn(CN)2 + 2 NaCl
Zinc cyanide is white. It is insoluble in water but dissolves readily in dilute acids to form the salt and hydrocyanic acid:
Zn (CN) 2 + 2HCl = 2 HCN + ZnCl2
It dissolves in alkalies to form double cyanides and zincates:
2Zn(CN)2 + 4 NaOH = Na2Zn(CN)4 + Na2ZnO2 + 2 H2O
2Zn(CN)2 + 2Ca(0H)2 = CaZn(CN)4 + CaZnO2 + 2 H2O
Alkaline cyanides dissolve zinc cyanide to form double cyanides:
Zn(CN)2 + 2 NaCN = Na2 Zn(CN)4
SODIUM ZINC CYANIDE Na2Zn(CN)4:
This complex is formed similarly to Zn(CN)2 except that twice as much alkaline cyanide is used:
ZnCl2 + 4 NaCN = NaZn(CN)4 + 2 NaCl
Sodium zinc cyanide is soluble in water dissociating to some extent as follows:
Na2Zn(CN)4 <–> 2Na+ + Zn(CN)4 <–> Zn (CN)2 + 2 CN-
This dissociation is said to be increased in the presence of free alkali. The addition of mineral acid to solutions of sodium zinc cyanide first precipitates zinc cyanide:
NaL2Zn(CN)4 + 2 HCl = Zn(CN)2 + 2 HCN + 2 NaCl
and then, as more acid is added, the zinc cyanide is decomposed to hydrocyanic acid. In pure solutions the zinc cyanide is precipitated quantitatively at a pH of 4 to 5 (indicated by methyl orange) by the addition of hydrochloric acid. As soon as the pH falls below 4 the zinc cyanide decomposes rapidly to zinc chloride and hydro-cyanic acid.
ZINC FERRO-CYANIDE Zn2Fe(CN)6: This complex is prepared by adding a soluble ferro- cyanide to a solution of a zinc salt:
2 ZnCl2 + Na4Fe(CN)6 = Zn2Fe(CN)6 + 4NaCl
Zinc ferrocyanide is a white salt, insoluble in water and dilute acids. It is soluble in alkalies forming sodium ferrocyanide and sodium zincate:
Zn2Fe(CN)6 + 8 NaOH = Na4Fe(CN)6 + 2 Na2ZnO2 + 4 H20
It dissolves in alkaline cyanide solutions to form sodium ferrocyanide and sodium zinc cyanide:
Zn2Fe(CN)6 + 8 NaCN = Na4Fe(CN)6 + 2Na2Zn(CN)4
Solubility of Metallic Zinc in Cyanide Solutions
Researchers investigated the solubility of commercial zinc dust in cyanide solutions under various conditions. This zinc dust contained about 90 percent metallic zinc, the greater part of the remainder being zinc oxide; it was all minus 150 mesh. Their experiments showed that commercial zinc dust is readily soluble in fresh cyanide solution; increasing the time of agitation increases the amount dissolved, provided that an excess of cyanide is present. Solutions containing no lime dissolve more zinc in a given period than solutions containing lime. For example, 1200 ml. of solution analyzing 0.145 percent NaCN and with no lime present dissolved 0.47 gram of zinc out of a total of 0.5 gram in 2 hours at 23°C; a similar solution containing 0.05 percent CaO dissolved 0.352 gram of zinc during the same period.
Zinc dissolves in cyanide solutions whether or not oxygen is present according to the equations:
2 Zn + 8 NaCN + O2 + 2 H2O = 2 Na2Zn(CN)4 + 4 NaOH
Zn + 4 NaCN + 2 H2O = Na2Zn(CN)4 + 2 NaOH + H2
The relative rates at which these reactions proceed are not known.
Solubility of Zinc Minerals in Cyanide Solutions
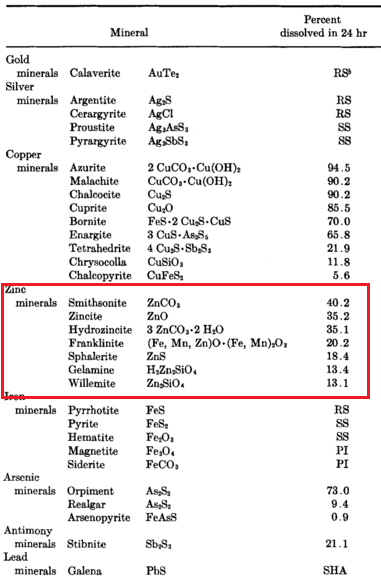
The same Researchers determined the relative solubilities of zinc minerals in cyanide solutions. Specimen zinc minerals were ground to minus 100 mesh and mixed with finely ground clean quartz in such proportions that each mixture contained about 1.25 percent zinc. Each mixture was agitated in cyanide solution containg 0.197 percent NaCN and free alkali (the particular alkali was not stated) for 24 hours at 45 °C. The ratio of solution to ore was 5 to I.
The results show that every zinc mineral dissolved in cyanide to an appreciable extent, from 40.2 percent of the total with smithsonite down to 13.1 percent with willemite. Calculating from the experimenters’ data, the amount of zinc dissolved in the case of smithonite would mean that the cyanide solution contained about 0.10 percent zinc, and the solution from the willemite mixture 0.03 percent zinc at the end of the tests. These amounts were considered sufficient to affect the dissolving efficiency of cyanide solutions under the usual conditions for cyanidation.
When sphalerite dissolves in cyanide solution, the reaction representing the dissolution:
ZnS + 4 NaCN <—> Na2Zn(CN)4 + Na2S
is reversible and the extent .to which it proceeds to the right in the absence of oxygen will be pro-portional to the strength of the cyanide solution. The rate at which the sodium sulphide oxidizes will also influence the speed of the reaction. In water, sodium sulphide hydrolyzes almost en-tirely to the hydrosulphide and the hydroxide thus:
Na2S + H2O <–> NaSH + NaOH
In the presence of oxygen, sodium hydrosulphide and sodium sulphide are oxidized to sodium thiosulphate:
2 Na2S + 202 + H2O = Na2S2O2 + 2 NaOH
2 NaSH + 202 = Na2S2O3 + H2O and then to sulphate:
Na2S2O3 + 2 NaOH + 202 = 2Na2SO4 + H2O
Besides thiosulphate, other intermediate products such as sulphites and thionates may be formed in the process of oxidation. In the presence of sodium cyanide, sodium thiocyanate is formed as follows:
2 NaSH + 2 NaCN + 02 =
2 NaCNS + 2 NaOH
2 Na2S + 2 NaCN + 2 H2O + 02 =
2 NaCNS + 4 NaOH
In dilute cyanide solutions it is probable that all of the above reactions take place and that when sphalerite dissolves in cyanide, the solution will contain thiocyanate, sulphate and intermediate sulphur compounds, in addition to double zinc cyanide. All of these sulphur compounds, with the exception of the end products of the reactions, thiocyanate and sulphate, will consume oxygen during their decomposition. These products, therefore, will be competing for the oxygen necessary for the dissolution of the precious metals. This means that in order to maintain oxygen in solution to dissolve the precious metals, the intermediate sulphur products formed during the decomposition of sphalerite in cyanide have to be oxidized as rapidly as they are formed. Thus, in the presence of zinc sulphide, an undesirable cycle has to be maintained to insure oxygen in the cyanide solutions, in which more zinc is dissolved, more cyanide is consumed, and more oxygen consuming compounds are formed.
The dissolution of oxide zinc minerals in cyanide solutions is less complicated:
ZnO + 4 NaCN + H2O = Na2Zn(CN)4 + 2 NaOH
ZnCO3 + 4 NaCN = Na2Zn(CN)4 + Na2C03
Zn2Si04 + 8 NaCN + H2O =
2 Na2Zn(CN)4 + Na2SiO3 + 2 NaOH
It may be noted that the oxide minerals dis-solve in cyanide to form an alkali in addition to forming sodium zinc cyanide. This alkali, and the protective alkali in the cyanide solution, also react with oxide zinc minerals. Where the alkali is sodium hydroxide, soluble sodium zincate is formed:
ZnO + 2 NaOH = Na2ZnO2 + H2O
With lime the reaction would be:
ZnO + Ca(OH)2 = CaZnO2 + H2O
although very little is known of the actual composition, true formula, or solubility of calcium zincate.
