When heat is applied to a cyanide solution containing metallic gold, two opposing factors affect the rate of dissolution. The increase in temperature would be expected to increase the activity of the solution and thus increase the rate of dissolution of gold. At the same time the amount of oxygen in the solution would decrease because the solubility of gases decreases with increasing temperature.

Julian and Smart determined the solubility of gold in 0.25 percent KCN solution at temperatures between 0°C and 100°C. They found that the rate of dissolution increased to a maximum at 85 °C although the oxygen content of the solution at this temperature was less than half of that at a temperature of 25°C. In addition, they found that at 100 C the rate of dissolution of gold was only slightly less than the maximum although the solution contained no oxygen. The explanation offered for this departure from what is considered the normal reaction for the dissolution of gold, i.e., where oxygen is considered essential, is that the capacity of an electrode to absorb or retain hydrogen at its surface is less in a heated solution than in the cold. Thus, the maximum opposing electromotive force due to polarization becomes less and less as the solution becomes heated, until the EMF of the dissolving gold overbalances polarization, and dissolution of the gold can proceed without oxygen. Thus, polarization can be prevented by oxygen which oxidizes the hydrogen at the surface of the gold and permits dissolution of gold at low temperatures; or, it can be prevented by heat which dislodges the hydrogen from the gold surface and permits the gold to dissolve without using oxygen. In the latter case the dissolution of gold should be accompanied by an evolution of hydrogen. Thus, Janin’s equation for the dissolution of gold in cyanide solutions might be substantially correct for hot solutions.
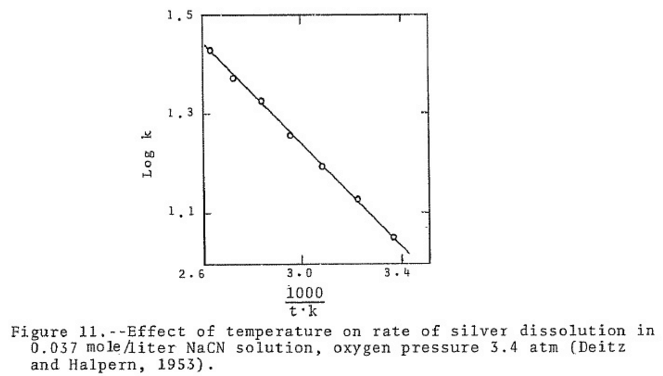
In practice the use of hot solutions for the extraction of gold from an ore has many disadvantages such as the cost of heating the ore and solution, the increased decomposition of cyanide due to heat, the increased consumption of cyanide due to the accelerated reaction between cyanicides in the ore such as sulphides of copper, iron and cyanide.
https://www.academia.edu/292086/Kinetics_and_Mechanism_of_Gold_and_Silver_Dissolution_In_Cyanide_Solution
