Thiourea Silver Leaching

Unlike base metals which are readily soluble in mineral acids, noble metals like gold and silver require the presence of oxidizing and complexing agents for dissolution. Silver approaches base metal behavior in acidic thiourea solutions (Ag + 3Thio ↔ Ag(Thio)3+ + e, E° = 0.025 V) requiring only mild oxidizing conditions for dissolution. Accordingly, the following […]
Zinc Box Gold Precipitation

The simplest precipitation equipment for a small mine is what is known as a zinc-box (Fig. 150). This consists of a long, narrow, sloping, wood or painted sheet-iron box divided into wide compartments or cells which are separated by baffle-boards and narrow compartments. The bottom of the box slopes to one side to facilitate the […]
Cyanidation
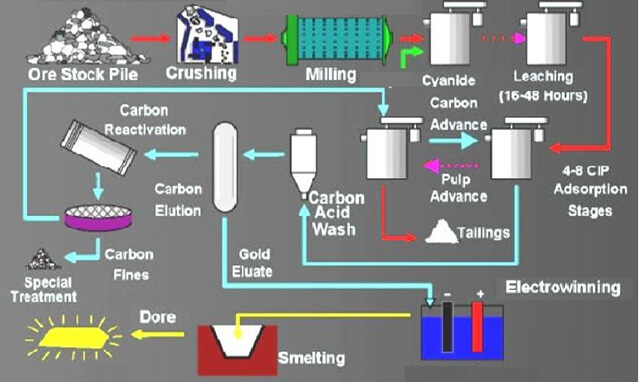
As coarse gold is not dissolved by cyanide, it must be removed from an ore by one of the methods already described. Weak solutions of sodium cyanide (or potassium cyanide, seldom used) will dissolve the fine, untarnished gold and silver in ores and tailings, provided the latter have been ground fine enough and do not […]
Precipitation of Copper contained Silver leached out with Copper

Having refractory ore under treatment, it is generally the case that copper is also found in it. While roasting, the presence of copper is favorable for the chlorination of the silver, but copper ores require some more salt, especially if it is intended to save the copper also. The more chloride of copper formed, the […]
Zinc Box Construction and Operation
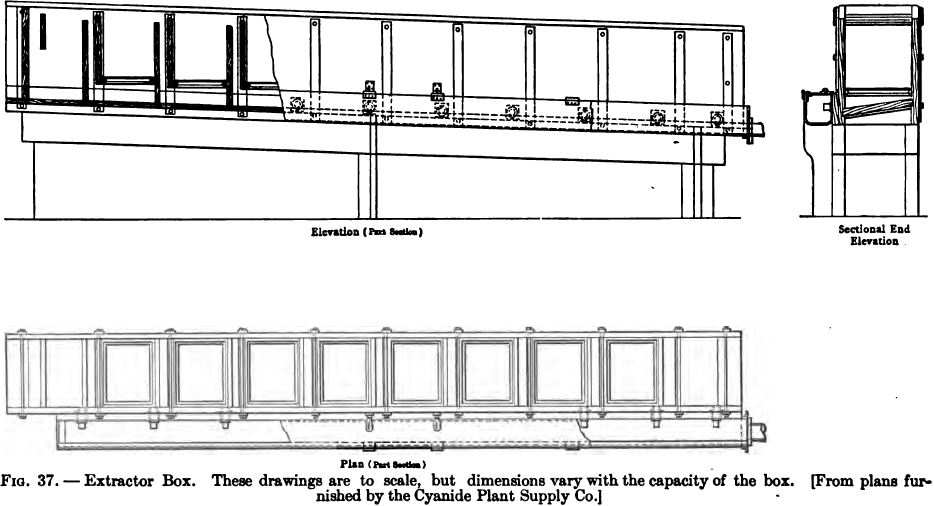
The precipitation by zinc shavings is carried out in rectangular boxes or troughs, divided by transverse partitions into a number of compartments. The partitions are so arranged that the solution flows alternately downward through a narrow compartment, and upward through a wide one, the latter alone containing the zinc shavings. The height of every alternate […]
Ferrocyanides

The study and use of ferrocyanides was initiated with the discovery of the pigment “Prussian Blue” by Diesbach in 1704. Thus, they are among the earliest commercial chemicals and have been produced in large quantities for many years. In the United States, American Cyanamid Company, with its ample supplies of cyanides as starting materials, has […]
Batch Cyanidation Leaching of Flotation Concentrate
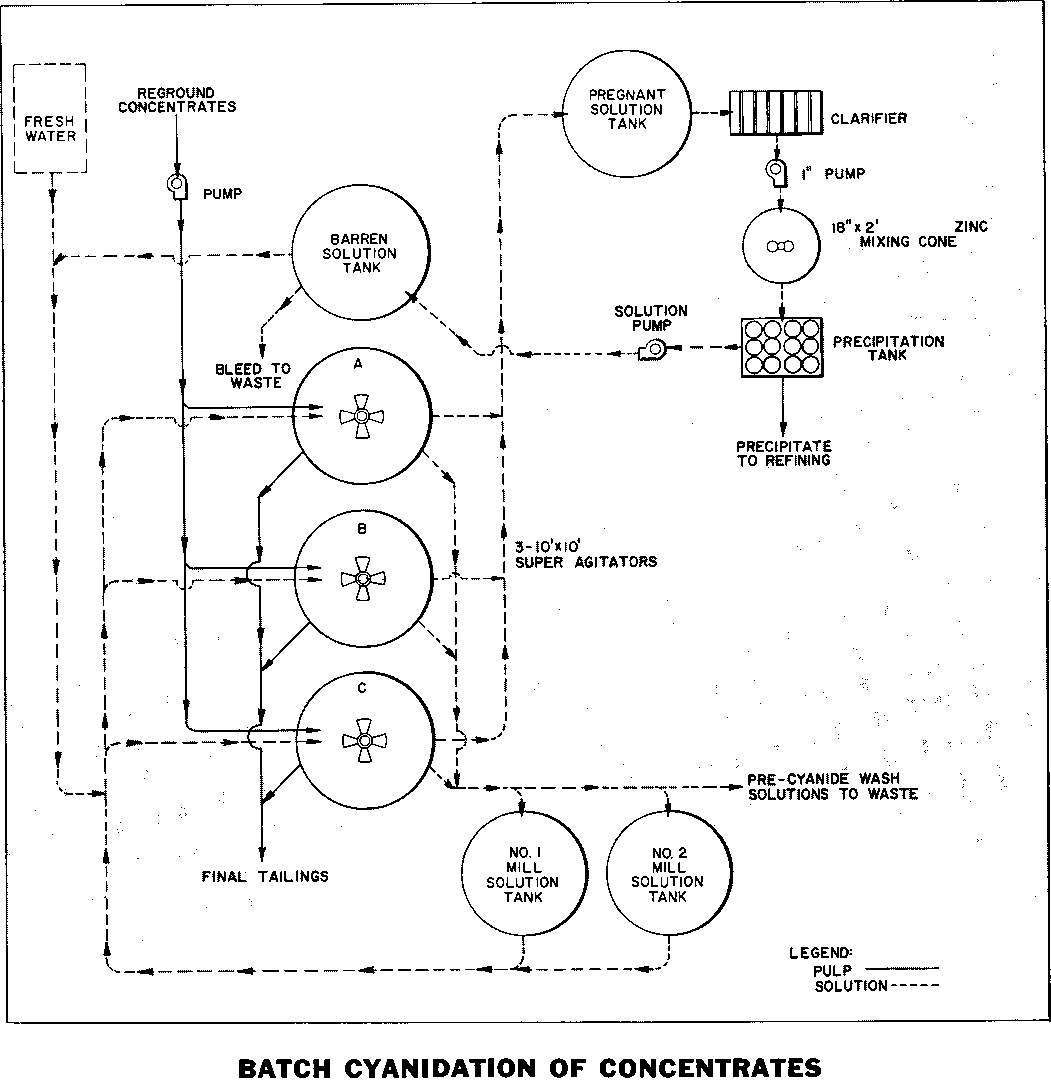
The production of gold bullion from gravity or flotation concentrates is often an important economic consideration for an isolated gold mining operation. It is assumed in this case that the coarse free gold has been recovered by the Mineral Jig in the grinding circuit and that the jig concentrate has been amalgamated. This treatment produces […]
Cyanide Destruction Hypochlorite / Chlorine
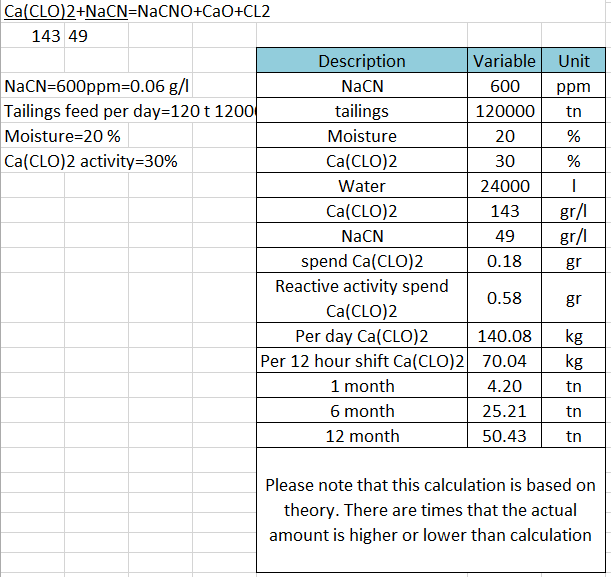
In the Cyanide Destruction by Hypochlorite reaction, the pH has a strong inverse effect on the ORP. Thus, wastewater treatment facilities must closely control the pH to achieve consistent ORP control, especially if they use hypochlorite as the oxidizing agent. Adding hypochlorite raises the pH, which, if unchecked, lowers the ORP. calling for additional hypochlorite. Controlling […]
Extraction Of Silver

The extraction of silver by the solving processes simple. The ore is first roasted with salt in the usual way, whereby the formation of base metal chlorides cannot be avoided entirely. After roasting, the ore is first subjected to leaching with water, in order to extract the base metal chlorides, and then with hyposulphite of […]
Merrill Crowe Leach Solution Sampler

This keeps the leach sample secure prior to its assaying. A simple electric box, lock and key, piece of pipe, funnel, bottle and yes, some wire.
