Gold Compounds
Gold is characterised chemically by an extreme indifference to the action of all bodies usually met with in nature. Its simpler compounds are formed with difficulty, and decompose readily, especially when heated. The result is that gold is found in nature chiefly in the metallic form, and the mineralogist has, therefore, few compounds to consider. […]
Purple of Cassius Preparation
Purple of Cassius was discovered by Cassius of Leyden in 1683. It contains gold and oxide of tin, and is used to colour glass and glazes, various shades of violet, red and purple being thus obtainable. Several methods of preparation are used, of which the following is that employed at the factory at Sevres: Half […]
Gold Silicates

The existence of auro-silicates is now admitted without dispute, and gold has for centuries been used to impart colour to glasses, the method used being as follows: A solution of chloride of gold is added to a mixture of sand with alkalies and alkaline earths or lead, and the whole is then fused, and colourless […]
Gold Sulfite

Sulphites of Gold, alkaline sulphites, or sulphur dioxide, which reduce gold trichloride easily, do not produce the same effect on a solution of an alkaline aurate. If sodium bisulphite is added to a boiling solution of sodium aurate (NaAuO2) a yellowish precipitate is formed, soluble in excess of sodium bisulphite, and consisting of a double […]
Gold Oxides
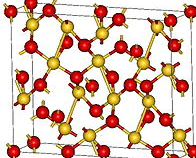
Aurous Oxide, Au2O This gold oxide is prepared by decomposing aurous chloride, AuCl, or the corresponding bromide by potash in the cold (Berzelius) when a violet precipitate forms, which is blackish when moist, but greyish when dry. When freshly precipitated it is soluble both in alkalies and in cold water, forming an indigo blue solution, with […]
Gold Cyanide Formulas

Cyanogen and gold unite in two proportions, forming aurous and auric cyanides, but the latter is only known with certainty in combination. Aurous Cyanide, AuCy, is obtained by heating aurocyanide of potassium, KAuCy2, with hydrochloric or nitric acid and washing with water. It is a lemon-yellow crystalline powder, insoluble in water, and unaltered by exposure […]
Gold Bromides

Gold Protobromide, AuBr, is a yellowish-green powder obtained by heating the tribromide to about 140°. It is insoluble in water, but is decomposed by it, metalic gold and the tribromide being formed; the change is especially rapid on boiling, and is hastened by the presence of hydrobromic acid. Auro-auric Bromide, Au2Br4, is produced by the […]
Gold Chloride
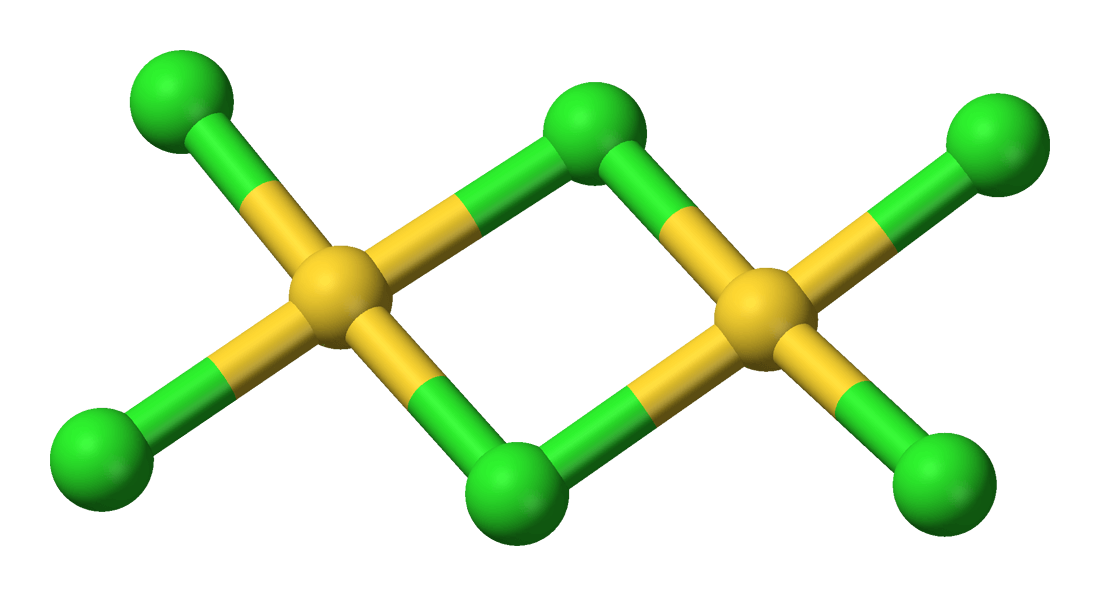
Gold Monochloride or Aurous Gold Chloride “AuCl” is a salt is prepared by heating the trichloride to 185° in air for twelve hours. It is non-volatile and unaltered at ordinary temperatures and pressure by dry air, even when exposed to light, but begins to decompose at temperatures above 160°, and the decomposition is complete if it is […]
