Separate Nickel and Cobalt by Electrorefining
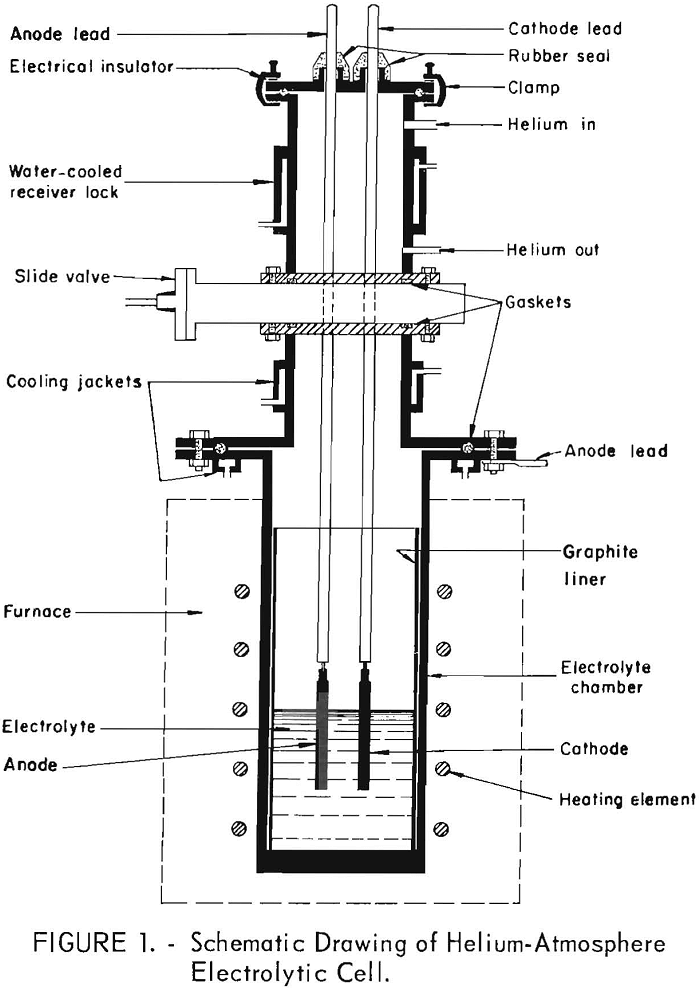
The electrolytic separation of nickel and cobalt by electrorefining in a molten salt electrolyte was shown to be technically feasible. Nickel-cobalt alloys containing up to 5.5 percent cobalt were electrorefined in a KCl-LiCl-NiCl2 electrolyte to a high-purity nickel metal meeting atomic energy specifications. The amount of cobalt transferred to the cathode deposit was found to […]
Amalgam Electrorefining of Zinc & Tin
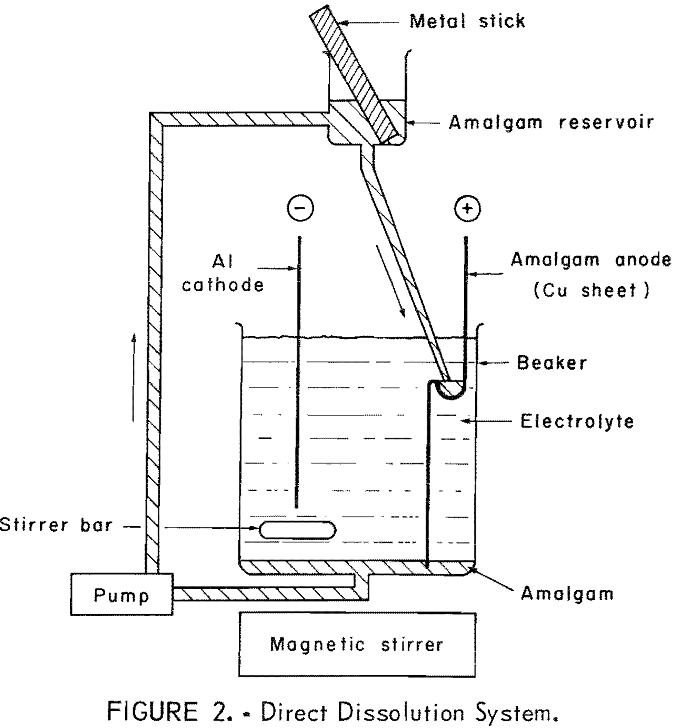
The following conclusions are drawn from the results of the laboratory tests of amalgam electrorefining of zinc and tin: Amalgam electrorefining is a method by which these metals may be refined to total metal impurity concentrations of less than 5 ppm in zinc and 9 ppm in tin. The suitability of metal for amalgam refining […]
High Temperature Electrostatic Precipitator
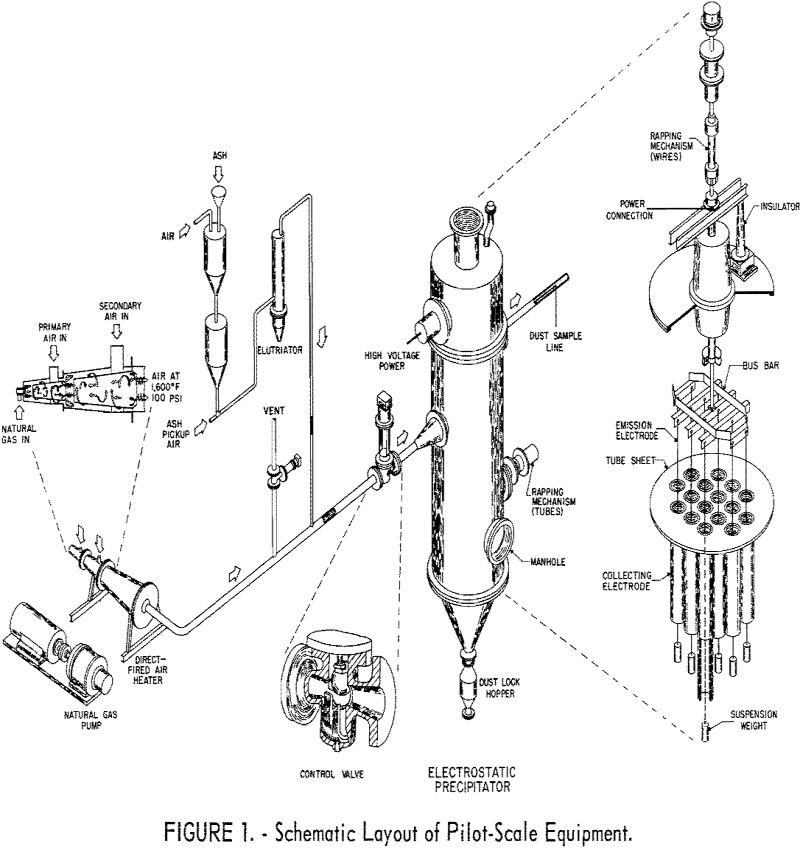
Industrial precipitators utilizing negative corona operate at maximum voltage to attain highest efficiency in removing finely divided particles from gas streams. Usage, however, is limited to applications at temperatures to about 1,000° F. Therefore, in conjunction with other work for processing coal at high temperatures, the Bureau of Mines sought to extend the applicability of […]
Recover Metals from Electroplating Wastes
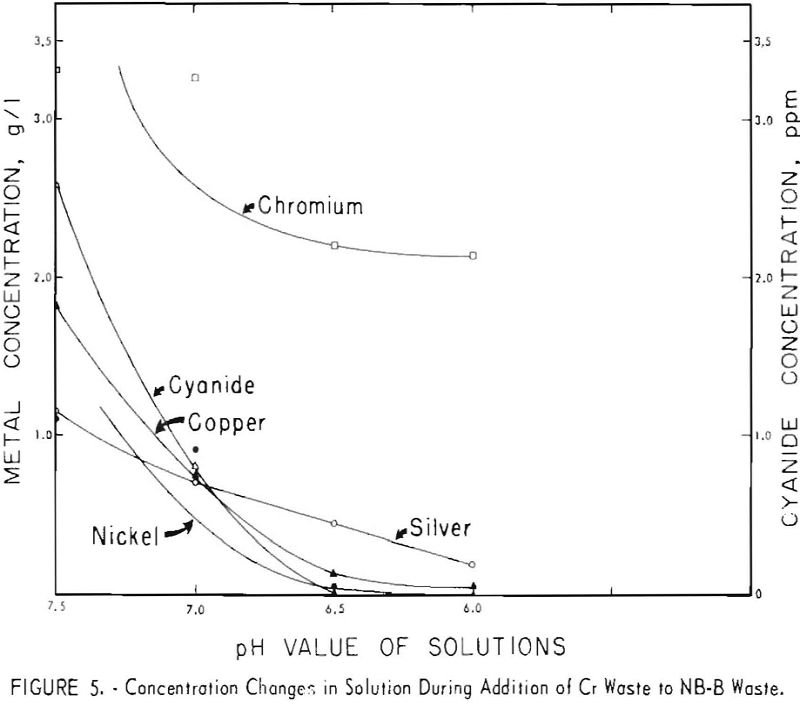
The waste-plus-waste treatment method can be successfully used on a variety of acid and alkaline electroplating wastes. The method is economically attractive because of the low cost of the waste-plus-waste addition step: No additional reagents are necessary, and the metal values are concentrated. In the five waste-plus-waste combinations investigated, combination of the two wastes caused […]
Recover Mercury by Electrolytic Oxidation
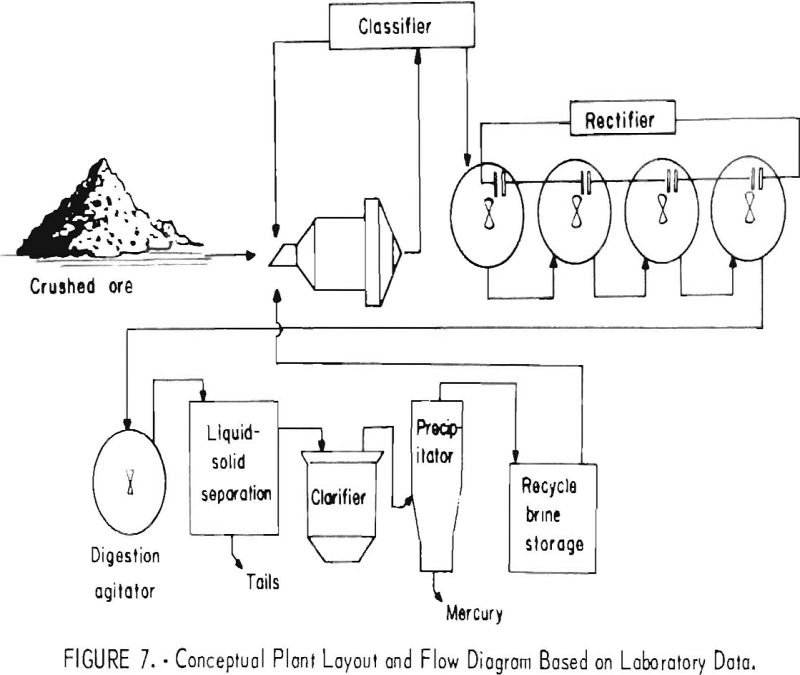
An aqueous oxidation treatment process developed by the Bureau of Mines has been shown to be effective for treating carbonaccous gold ores to improve gold recovery. Ozone, chlorine, sodium hypochlorite (NaOCl), and calcium hypochlorite [Ca(OCl)2] were all shown to be effective reagents for oxidizing the carbonaceous material in the ore. However, generation of oxidizing conditions […]
Tungsten Metal & Tungsten Carbide Electrolysis
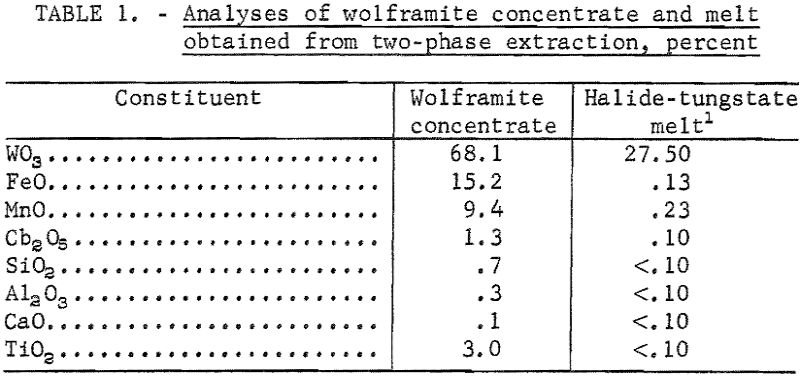
A high-temperature, two-phase extraction technique has been developed for the recovery of tungstic oxide (WO3) from tungsten-bearing minerals. The technique consists of dissolving scheelite (CaWO4) or wolframite in a system of two immiscible liquids-sodium chloride and sodium silicate-at 1,080° C. Tungstic oxide is converted to sodium tungstate (Na2WO4 ) and is transferred to the upper […]
Ion Exchange Resin to Recover Gold from Acid Solution
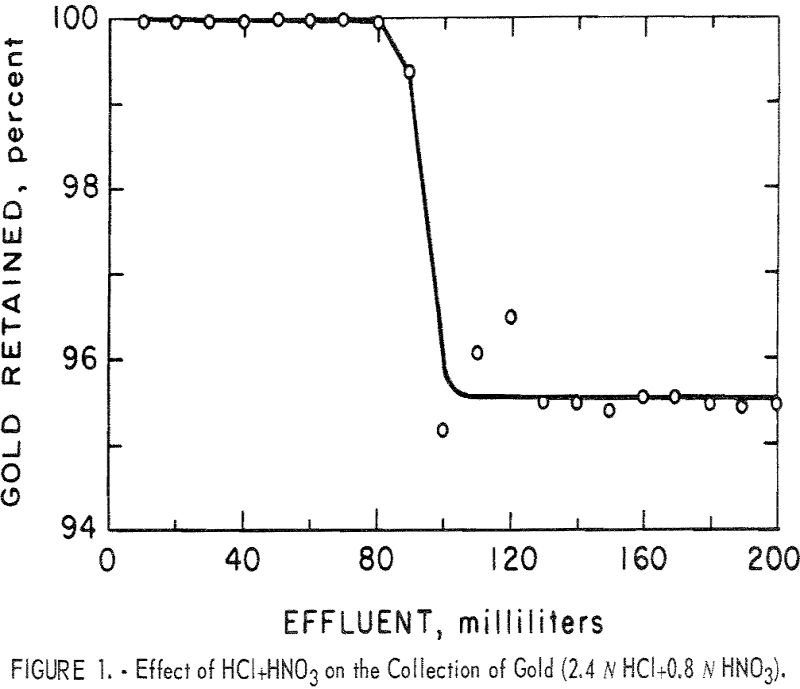
An ion exchange resin was found to quantitatively and selectively collect gold from acid solutions over a wide range of acid concentrations. Common metals at high concentration are not collected by the resin and they do not seriously interfere with the collection of gold. Alkaline cyanide solutions gradually destroy the capability of the resin to […]
Recover Aluminum by Electrowinning
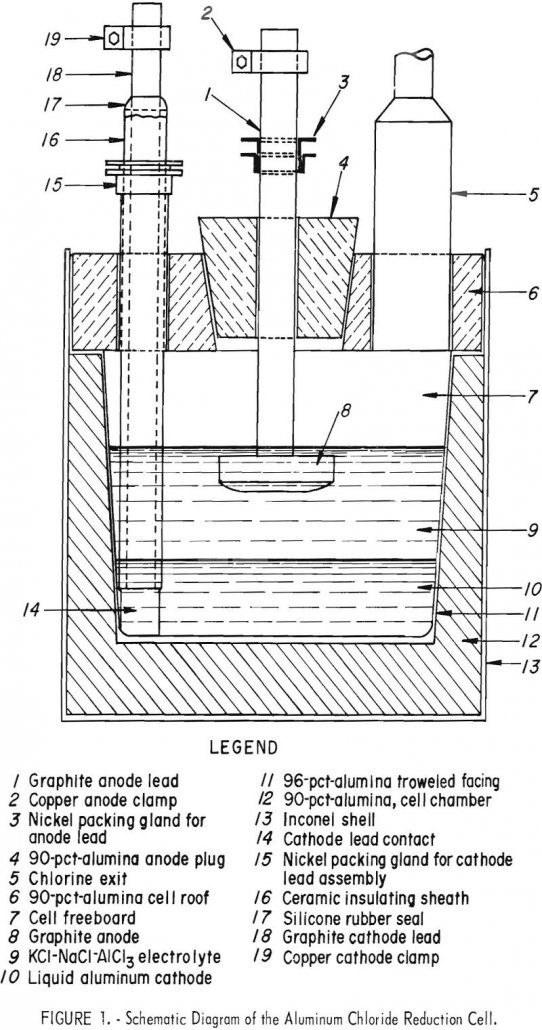
The preparation of aluminum by molten salt electrolysis of AlCl3 was undertaken as part of the Bureau of Mines overall research program to develop methods for the utilization of domestic low-grade raw materials, de Beauchamp and Thomas and others summarized the preparation and manufacture of anhydrous AlCl3 from a variety of aluminiferous materials: aluminas, bauxites, […]
Electrowinning MischMetal
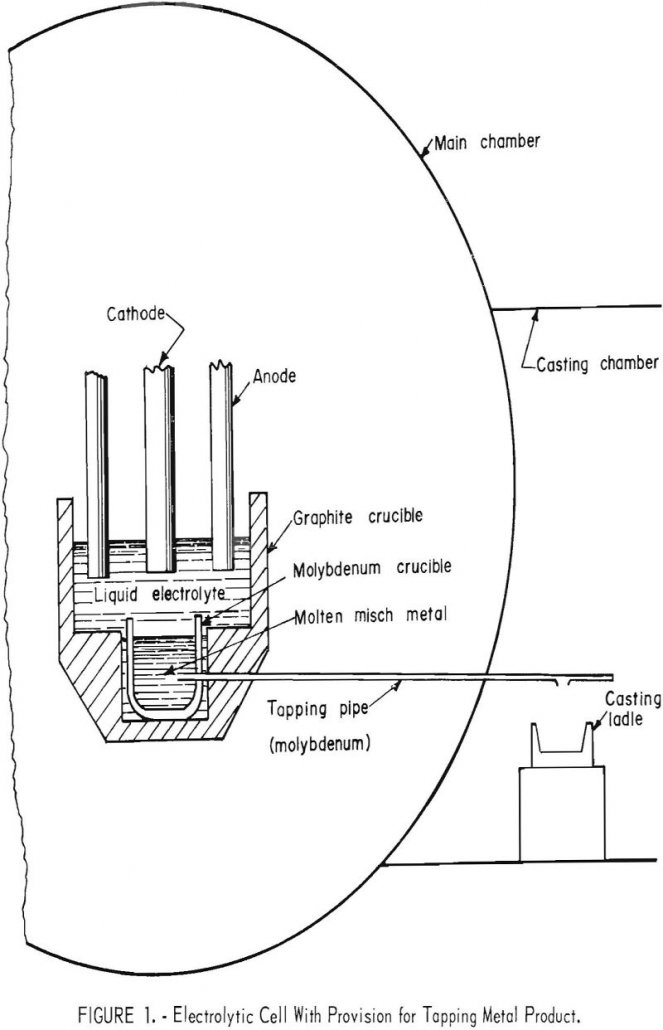
For many years, we conducted a research program on electrolytic preparation of rare-earth metals. Cerium, lanthanum, and neodymium, praseodymium, and didymium metals have been electrowon from their respective oxides dissolved in fluoride melts. Commercial production of mischmetal, a mixed rare-earth metal consisting essentially of cerium, lanthanum, neodymium, and praseodymium, is presently limited to electrolysis of […]
Electrowinning Tungsten
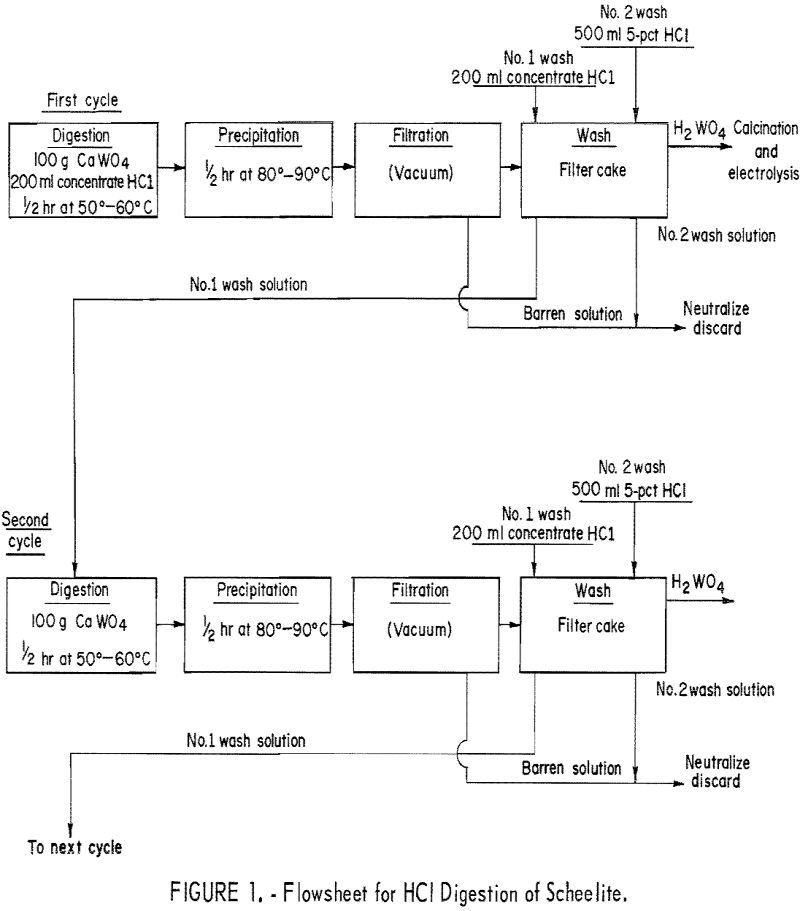
We demonstrated techniques for the electrowinning of tungsten directly from scheelite concentrate. The major problem with direct electrowinning is the accumulation of lime (CaO) in the electrolyte. Scheelite mineral concentrates usually contain 60 to 70 weight-percent WO3 and about 20 weight-percent CaO. The results of the CaO buildup are decreased metal purity, increased electrolyte viscosity, […]
