Nitric Sulfuric Leach of Copper Concentrate
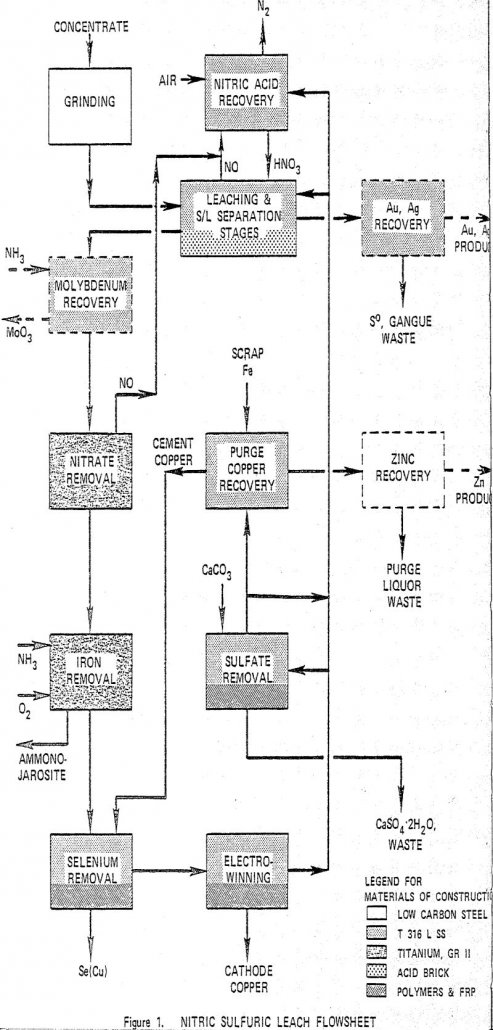
Copper is leached in a staged reactor system utilizing nitric and sulfuric acids at 105°C. Iron is removed from the pregnant liquor as a jarosite and cathode copper is electrowon directly from the purified pregnant liquor. The spent electrolyte is recycled. Nitrogen oxides evolved from the leach are reconverted to nitric acid and are also […]
Liquid Ion Exchange Fractionation of Metal Ions
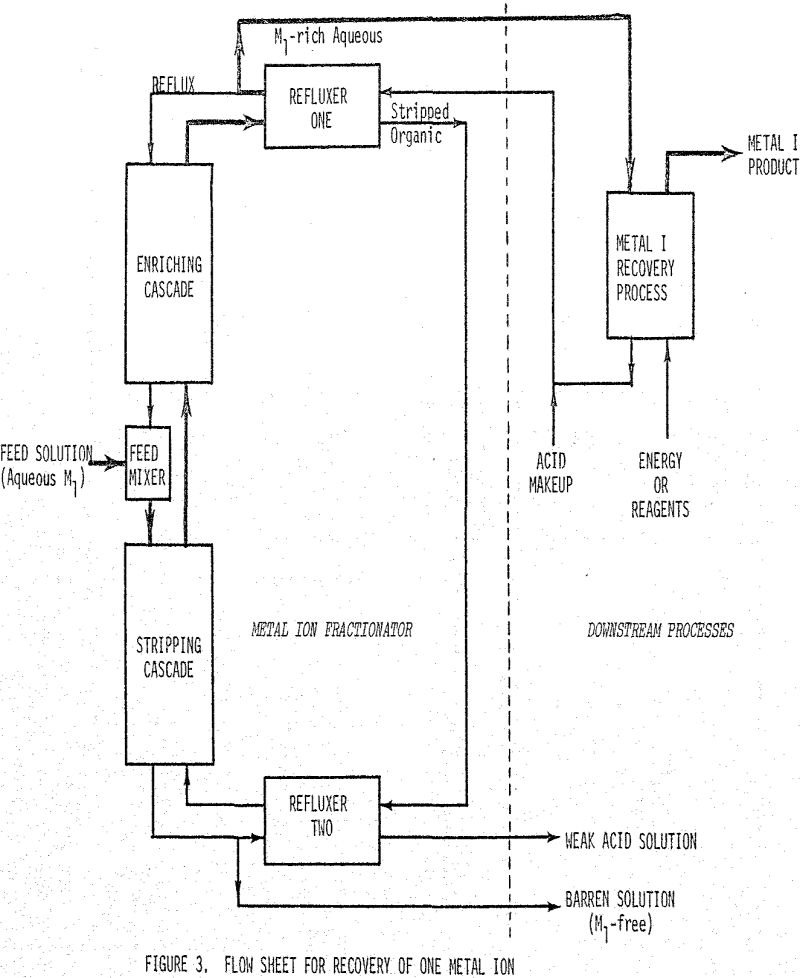
There are two basic problems when one attempts to separate a solution of metal ions utilizing a chelation-solvent extraction cascade (commonly called liquid ion exchange). First, it is impossible to produce any great degree of separation unless the separation factor (also called selectivity, which is the ratio of the distribution coefficients) is very large. Second, […]
Extraction of Nickel and Cobalt from Acidic Solutions
LIX63 in combination with DNNS selectively extracts nickel and/or cobalt from acidic leach liquors with pH values as low as 1.0. Nickel/cobalt and cobalt/nickel selectivities are achieved by varying the relative concentrations of LIX63 and DNNS. Increasing LIX63 concentration retards both extraction and stripping rates while DNNS has the opposite effect. Slope analyses of equilibrium […]
The Effect of Hydrogen Peroxide on Leach Dump Bacteria
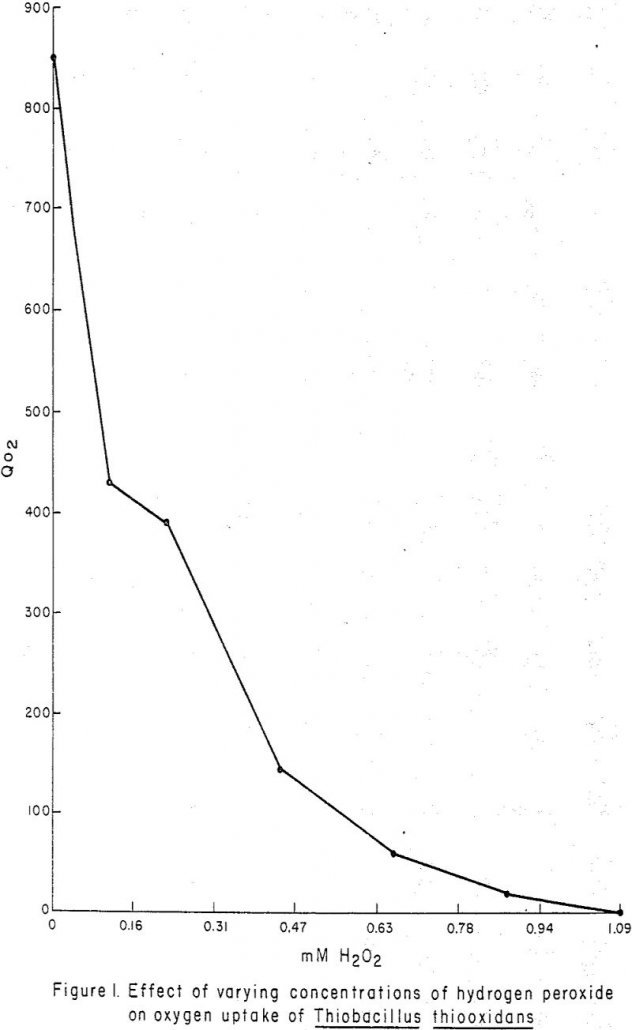
In recent years, hydrometallurgical processes for recovery of metal values from low-grade ore minerals have played an increasingly important role. Dump leaching of low-grade copper waste materials in the western United States now yields 11.5% of the total U. S. copper production. Although scavenger operations in uranium leaching have been successful, the state-of-the-art of in-situ uranium […]
Copper Sulfide Leaching by Ammoniac to recover Sulfur

Results of a preliminary study of the ammoniacal leaching of covellite (CuS) in an aqueous oxidizing system in the presence of a non-miscible organic sulfur solvent are described. Up to 60% of the sulfur can be recovered from the solvent as elemental sulfur while achieving high degrees of solubilization of the mineral. Experimental Leaching tests, […]
Hydrometallurgical Copper Extraction Process

The successful development of the Cyprus Copper Process has been an evolutionary series of events covering a time span of some seven years. What does the Cyprus Copper Process do? Very simply, it converts copper concentrates of varying composition into copper metal which has been proven to be equivalent in every way to electrolytic tough pitch […]
Sulfide Ion Leaching Kinetic of Wulfenite
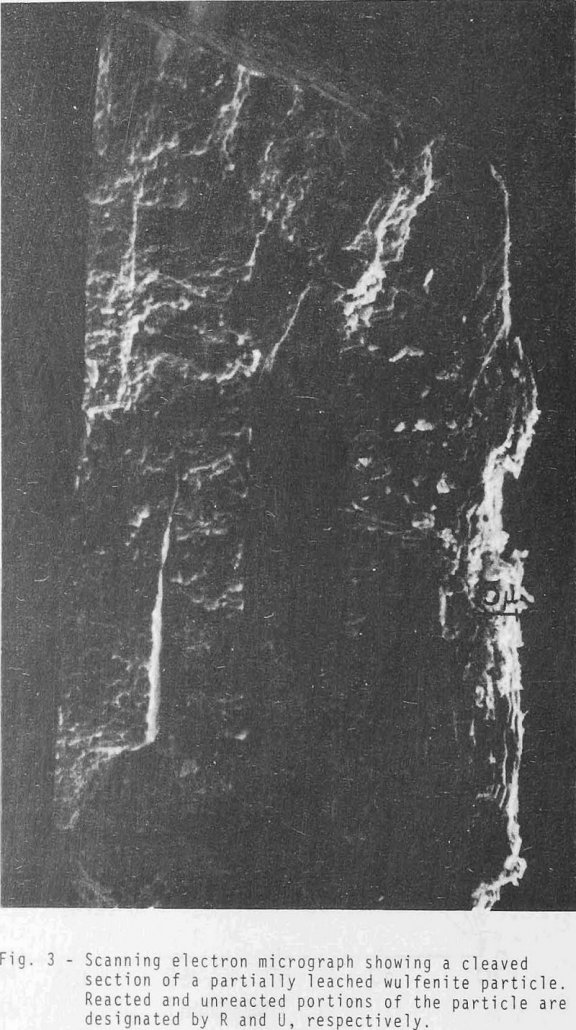
The principle oxide minerals of molybdenum are wulfenite (PbMoO4), ferrimolybdite (Fe2(MoO4)3·nH2O), powellite (CaMoO4), and ilsemannite (Mo3O4). Wulfenite is found in Arizona, New Mexico, Nevada, Utah, California, and Montana. It is also found in the states of Sonora and Chihuahua, Mexico. Prior to 1915 wulfenite ores were the primary source of molybdenum. All leaching experiments were […]
Hydrochloric Acid Copper Leaching
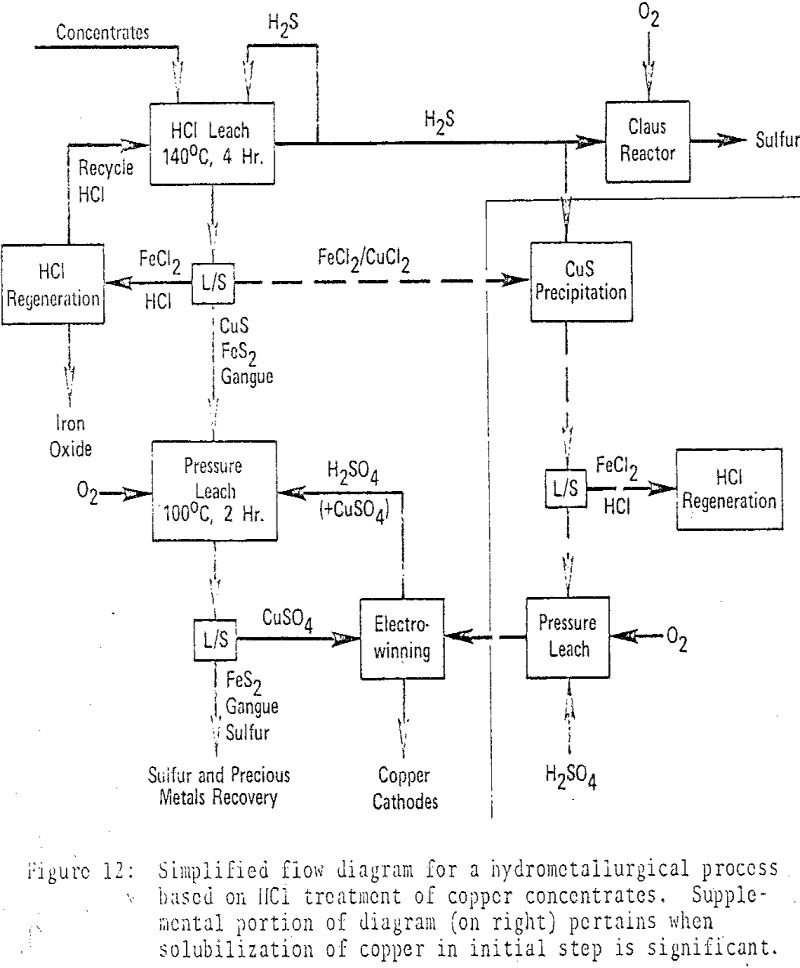
Experimentation with chalcopyrite concentrate in dilute hydrochloric acid, under reflux conditions (in Pyrex flasks) and with an inert purge gas, revealed substantial amounts of H2S production and iron solubilization, with negligible copper solubilization, after 2½-4 days. Under comparable condition the extent of reaction with dilute sulfuric, acetic and phosphoric acids was markedly less. Pertinent Chemistry […]
Recover Rhenium by Solvent Extraction
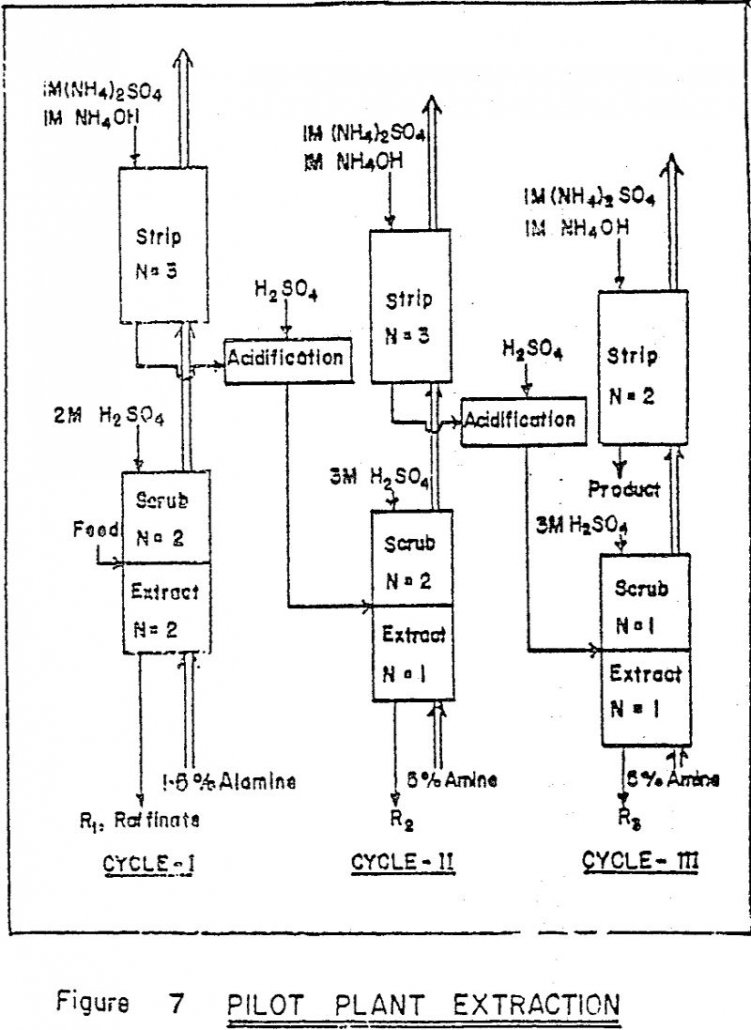
Rhenium is industrially recovered all over the world as a by-product of molybdenite processing. There are no separate deposits of rhenium minerals. Molybdenite concentrates, produced as co-products of porphyry copper deposits, contain 0.01 to 0.2% rhenium occuring as a substitutional impurity in the molybdenite lattice. Process Synthesis The solvent extraction process is integrated with other […]
How to Recover Rhenium from Uranium Leach Liquor
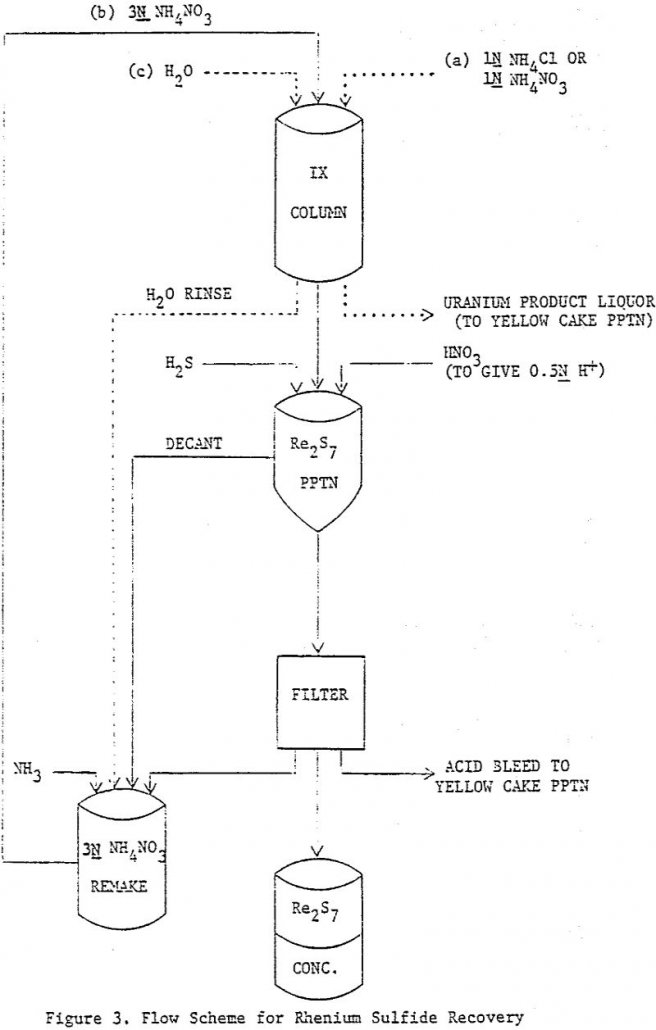
The discovery of rhenium on the ion exchange resin used at the uranium in-situ leach operations at Palangana (Texas) led to a laboratory study on the possible methods of recovery. Rhenium, present in small amounts in the ammonium carbonate leach liquors as the perrhenate anion, ReO4-, concentrated on the anion exchange resin, which was a […]
