Gold Cyanide Formulas

Cyanogen and gold unite in two proportions, forming aurous and auric cyanides, but the latter is only known with certainty in combination. Aurous Cyanide, AuCy, is obtained by heating aurocyanide of potassium, KAuCy2, with hydrochloric or nitric acid and washing with water. It is a lemon-yellow crystalline powder, insoluble in water, and unaltered by exposure […]
Gold Bromides

Gold Protobromide, AuBr, is a yellowish-green powder obtained by heating the tribromide to about 140°. It is insoluble in water, but is decomposed by it, metalic gold and the tribromide being formed; the change is especially rapid on boiling, and is hastened by the presence of hydrobromic acid. Auro-auric Bromide, Au2Br4, is produced by the […]
Gold Chloride
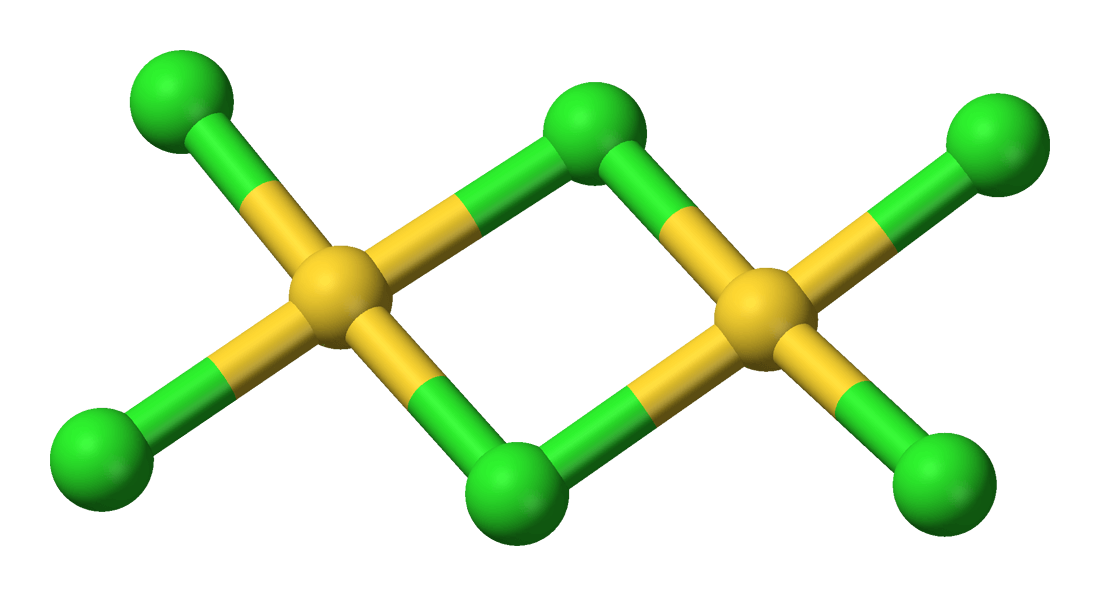
Gold Monochloride or Aurous Gold Chloride “AuCl” is a salt is prepared by heating the trichloride to 185° in air for twelve hours. It is non-volatile and unaltered at ordinary temperatures and pressure by dry air, even when exposed to light, but begins to decompose at temperatures above 160°, and the decomposition is complete if it is […]
How to Prepare a Sample for Assaying
Assaying has for its object the determination of the quantities of those constituents of a material which add to or detract from its value in the arts and manufactures. The methods of assaying are mainly those of analytical chemistry, and are limited by various practical considerations to the determination of the constituent of a small […]
How to Measure Specific Gravity
The relation of the weight of a substance to its volume should be kept in mind in all cases where both weight and volume are dealt with. Students are apt to imagine that on mixing equal volumes of, say, sulphuric acid and water, an acid of half the strength must be obtained. If the statement […]
Silver Assaying Methods
Silver is widely diffused, and has been found in most mining districts. It occurs native in sufficient quantity to constitute one of the chief ores of the metal. It also occurs combined with sulphur (as in argentite), with sulphur and antimony (as in stephanite or brittle silver ore, and in pyrargyrite or ruby silver), and […]
Gold Content Determination Methods
Gold occurs in nature chiefly as metal. It always contains more or less silver, and, in alluvial sands, may be associated with platinum and iridium. Gold is insoluble in hydrochloric or nitric acid, but is dissolved by aqua regia or by solutions of iodine, bromine, or chlorine. It is taken up by mercury, forming an amalgam, […]
Assaying Copper Determination Method
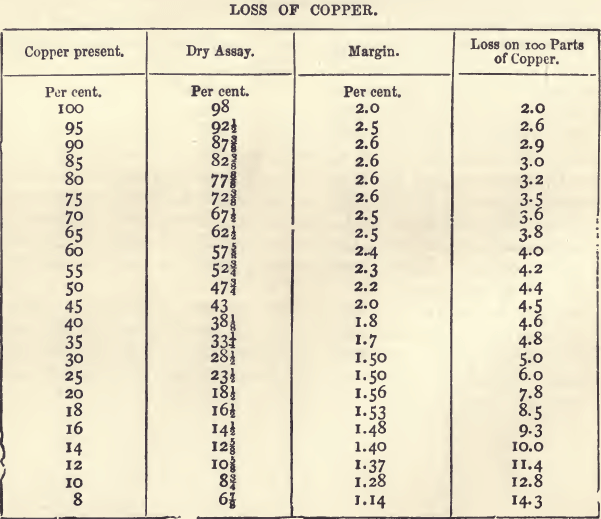
Copper occurs native in large quantities, especially in the Lake Superior district ; in this state it is generally pure. More frequently it is found in combination. The ores of copper may be classed as oxides and sulphides. The most abundant oxidised ores are the carbonates, malachite and chessylite; the silicates, as also the red […]
Assaying for Iron Determination Methods
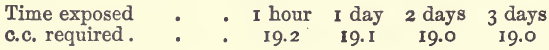
Iron rusts or oxidises very readily, and, consequently, is rarely found in the metallic state in nature; such native iron as is found being generally of meteoric origin or imbedded in basalt and other igneous rocks. It chiefly occurs as oxide, as in magnetite, haematite, and in the brown iron ores and ochres. Chalybite, which […]
Assaying Nickel Determination Methods

Nickel and cobalt are closely related in their chemical properties, and may best be considered together. Nickel is the commoner of the two, and is met with in commerce alloyed with copper and zinc as German silver; as also in the coinage of the United States and on the Continent. It is used for plating […]
