Determination of Acetates
Julian and Smart state that cyanide solutions undergoing decomposition form not only formates but acetates. Clennell states that he has never found acetates present in a working cyanide solution unless lead acetate was being used, but on the assumption that acetates may perhaps be formed in certain circumstances he suggests the following equation. 4KCN + 5H2O […]
Method to Assay for Soluble Sulphides in Leach Solution
This is seldom called for, but it happens occasionally that an appreciable amount of sulphide is developed in the cyanidation of concentrate, and some sulphide is sometimes apparent in the press effluent from aluminium precipitation which it may be desirable to estimate. For such small amounts Clennell recommends the colorimetric method. For this purpose a […]
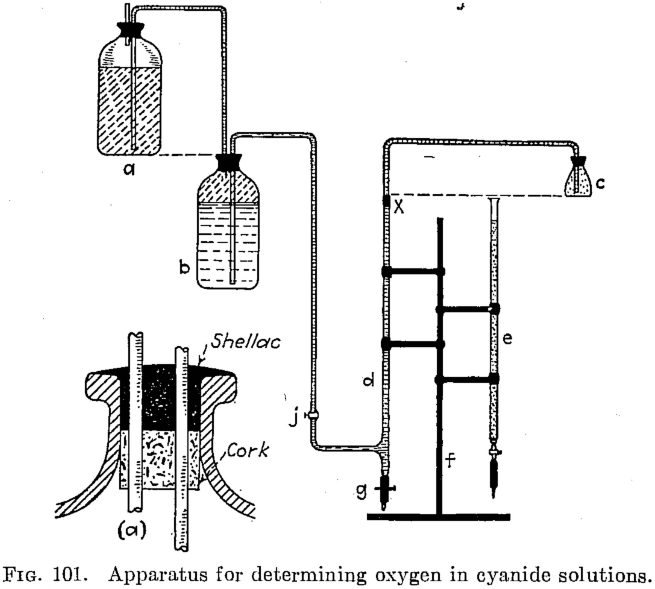
Determine Oxygen Content in Leach Solution

Here is a way how to determine the oxygen content during gold leaching and Estimation of available Oxygen Content in Cyanide Solutions. Until recently no simple and reliable method has been known for the estimation of free oxygen in working cyanide solutions so that the one here described supplies something that has long been needed. The matter […]
Assay Method of Gold or Silver in Leach Solution
Gold & Silver Assay Method No. 1 Evaporate a known quantity in a square boat made of lead foil, scorify, and cupel. This is not reliable for low grade solutions because the amount that can be taken for assay is too small. Gold & Silver Assay Method No. 2 Evaporate a known quantity in a large porcelain dish whose […]
Cyanide Reflux Distillation Apparatus
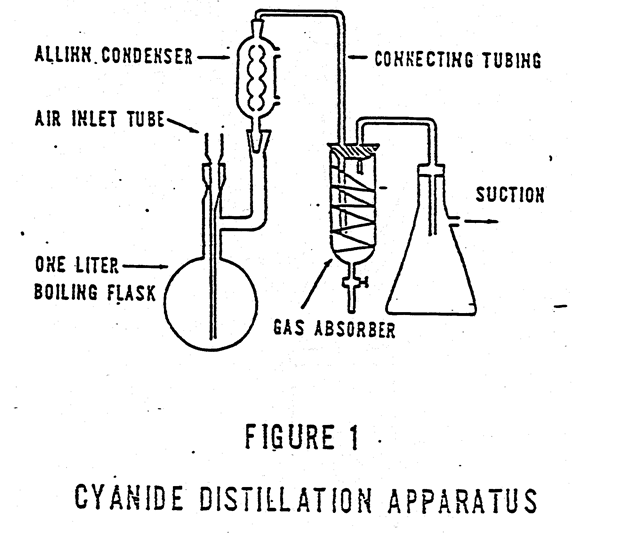
Distillation Procedure Samples without sulfide. Place 500 mL of the combined sample or an aliquot diluted to 500 mL in the 1 liter boiling flask. Pipet 50 mL of 1.25 N sodium hydroxide into the absorbing tube. If the apparatus in Figure 1 is used, add distilled water until the spiral is covered. Connect the […]
Assay gold and silver in cyanide solutions
In the assay of gold and silver in cyanide solutions the degree of accuracy and the speed desired are the governing factors in the choice of methods used and the quantity of solution taken for the determination. Evaporation {Litharge) Method To an evaporating dish add about 50 grams litharge and 146 to 292 cc cyanide solution. […]
Assaying for Zinc in Cyanide Solution
Zinc usually occurs in cyanide solutions as the double cyanide, but under certain conditions, e.g., in dilute solutions, a portion of the zinc may be present as zinc cyanide. It is possible that some may also exist as an alkaline zincate. Procedure To 500 cc of solution add 10 cc HCl, 10 cc HNO3, and […]
Analytical Method for Copper Content in Cyanide Solution
Short Iodide Method To 200 to 500 cc of solution add 10 cc HCl, 5 cc HNO3. Evaporate to about 50 cc, then cool, and add 8 cc H2SO4. Evaporate almost to dryness. Cool, add 5 cc water and 5 cc H2SO4, and again evaporate almost to dryness. Cool, add 50 cc water, and heat […]
Test for Traces of Cyanide
Here is a Qualitative Test Method to look for Cyanide Traces. To 500 to 1000 cc of the solution to be tested add 1 to 2 cc ammonium sulphide, (NH4)2S, and evaporate just to dryness. The final stages of evaporation should be done slowly. Cool, add 10 cc water, stir well, let settle, and filter. To the filtrate […]
Ferrocyanide Assay Determination
The most reliable method of determining ferrocyanide in a cyanide solution is to determine the total iron and calculate to ferrocyanide. Volumetric Method Procedure To 200 to 500 cc solution, depending upon the quality of ferrocyanide thought to be present, add 10 cc HCl and 5 cc HNO3, and evaporate to about 50 cc. Add […]
