Why is cyanide heap leaching used to extract gold from some deposits
Cyanide is used in heap leaching to extract gold from some low-grade deposits because of its ultra-low-cost and high effectivity are leaching gold.
Copper Gold Sulfide Ore – Flotation & Cyanidation Tests
Grinding the ore to minus 100-mesh gave satisfactory liberation of the minerals. Standard flotation procedure with potassium ethyl xanthate, cresylic acid, and lime produced good-grade copper concentrates with over 85 percent recovery of copper and about 60 percent recoveries of gold and silver. This stop was employed in two methods of treatment developed during laboratory testing. […]
Copper Leaching Practices
The first large-scale leaching and precipitation of copper was probably at Rio Tinto in Spain about 1752. The method employed comprised open-air water leaching of weathered piles of copper-bearing ore followed by precipitation of the copper by iron. A description of leaching and precipitation of copper at Rio Tinto in the 20th century is given […]
Sphalerite Leaching Kinetic
During roast, virtually all the iron in the concentrate feed is converted into zinc ferrite which remains almost inert in the neutral-leach stage. In order to recover zinc incorporated in ZnO·Fe2O3, a follow-up treatment of the leach residue is required. Ferric chloride is one of the ideal agent for the direct leaching of sphalerite. But […]
Activated Carbon & Cyanidation

In 1939 one of the authors described advances in carbon-cyanidation for the period 1932 to 1939 and included: (1) the dissolution of gold in an ore pulp by cyanide and its simultaneous adsorption by carbon, (2) the stage addition of adsorptive carbon and its movement countercurrent to the flow of the ore pulp, and (3) […]
Factors Affecting Ore Leaching
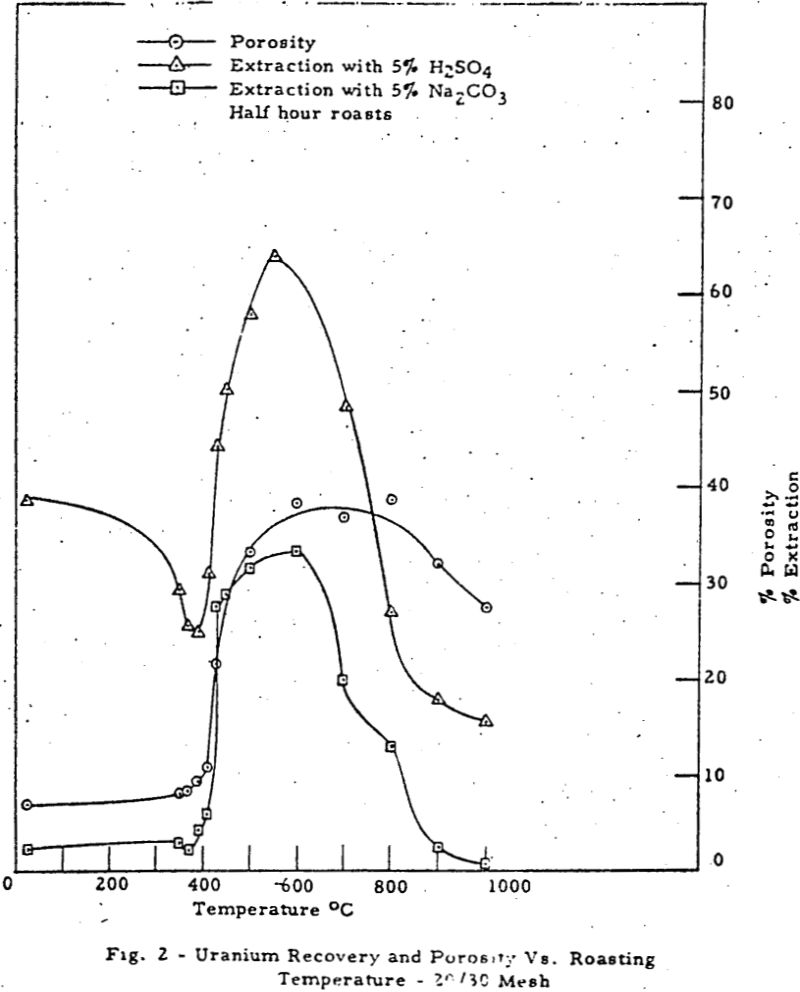
This work defines and systematizes the physical factors of the problem of leaching and demonstrates for the first tine the utility of macropore volume, micropore volume., specific surface, pore size distribution and permeability as parameters of leaching based on a study of the recovery of uranium from the Top Black member of Chattanooga Shale. A […]
How to Handle Coarse Ore in a CCD Thickener Circuit
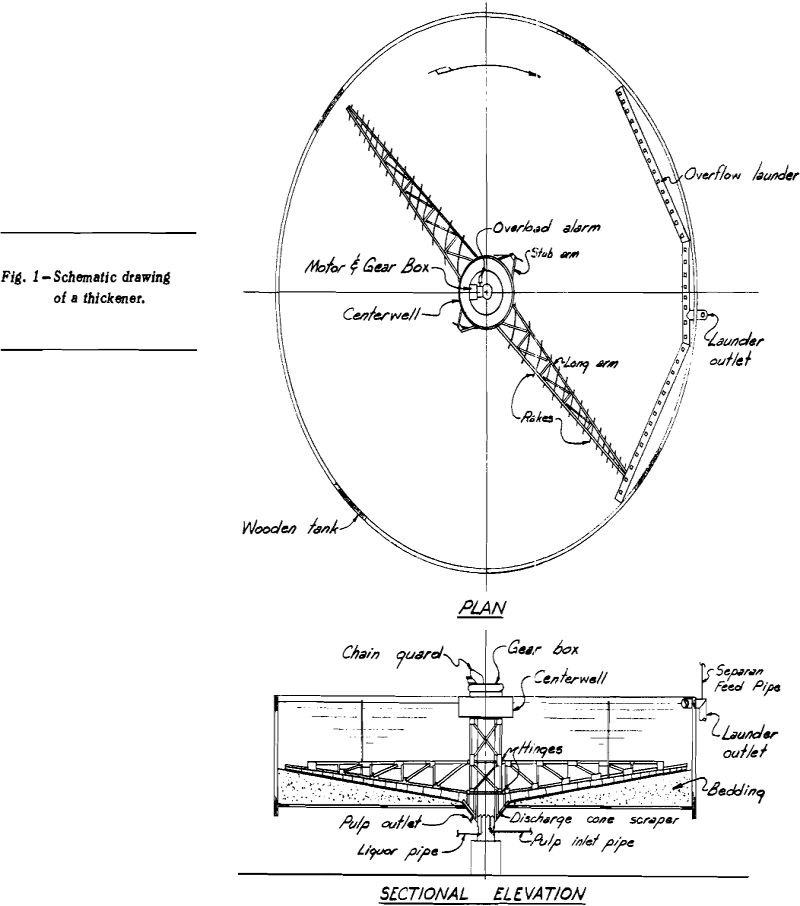
The use of a series of thickeners for washing of the slime fraction of a leached ore is not uncommon in the uranium industry; however, few ore processing mills have used a thickener circuit for washing leached total ore. The common practice is to wash the sand fraction in classifiers and the slime fraction only […]
Uranium Recovery with Advanced Acid Leaching Techniques
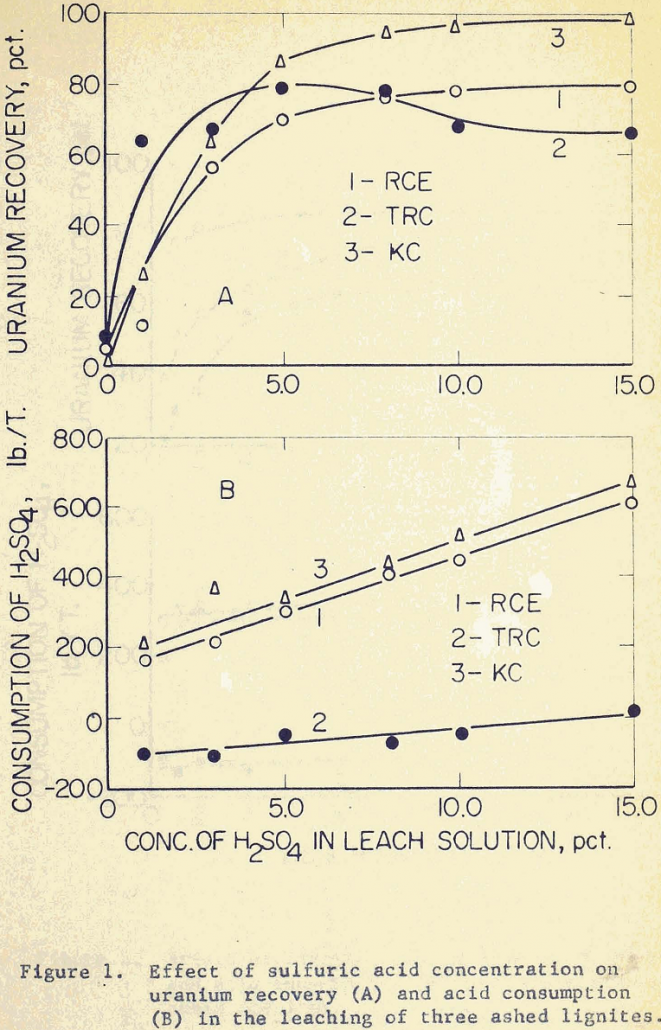
Compared to the uranium ores in Colorado Plateau and New Mexico, the uranium-bearing lignites in North and South Dakota are relatively difficult to process. This is due chiefly to the irregular mineralization of the lignites, and partly to the non-uniform distribution of uranium within the deposits. The three lignite samples used in this investigation were […]
Oxided Copper Ore Leaching
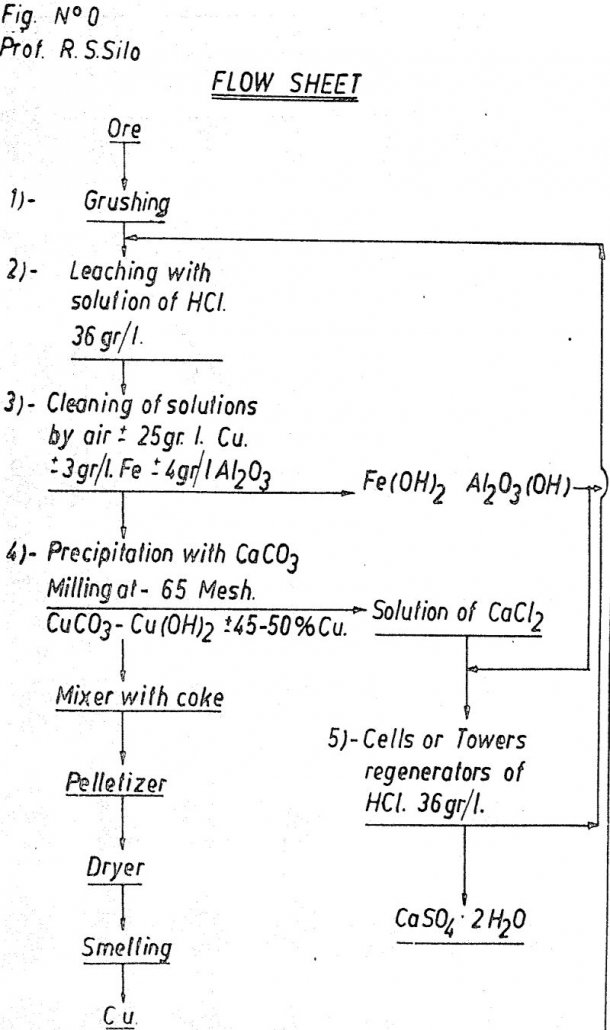
The principal characteristics of the process is that when applied in medium or in great degree, it only needs unelaborated raw materials such as sodium chloride and calcium carbonate, or of medium elaboration, as sulphur which is found in large quantity in the North of Chili however, the system may be also applied to small […]
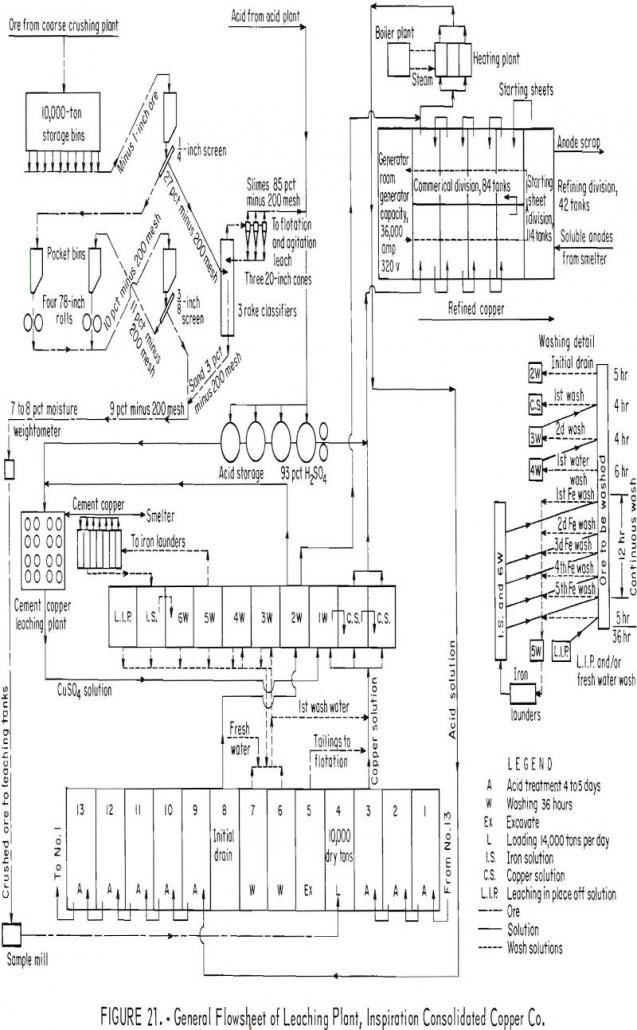
Heap Leaching Copper Ore
Ranchers began its evaluation of the Bluebird Mine in late 1963. The property which included some 400 acres, adjoined one of the country’s leading producers – Inspiration Consolidated Copper Company. Such proximity led many to equate availability with undesirability. However, a relatively short period of exploration and metallurgical evaluation and a simultaneous assessment of other […]
