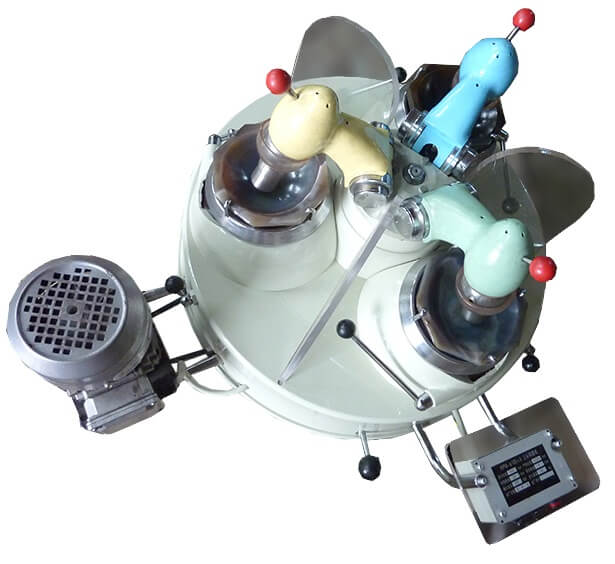
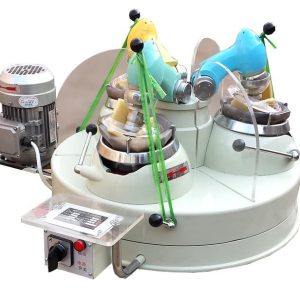
DESCRIPTION
A mortar and pestle are normally the only grinding option when the quantity of the material is limited, while mechanical mills usually require much larger amount of material to begin with. It is important to keep the milling equipment clean to prevent cross-contamination of samples. One of the best ways to clean a mortar and pestle before grinding a new sample is to use a 50:50 (by volume) mixture of nitric-acid (HNO3) and water. When using a hardened steel or a tungsten carbide ball-mill, the vial should be cleaned using a blaster with fine (<5um) AI2O3 powder, and the previously used balls should normally be discarded. Agate vials and balls can be cleaned using a 50:50 mixture of water and nitric acid. At the very’ least, flashing with a low-boiling point organic solvent (e.g., acetone or alcohol), wiping and drying the grinding equipment should always be performed.
One of the biggest concerns when using mechanical grinding in a ball mill is the possibility of contamination of the sample with the vial (balls) material. This is particularly true when hard materials are subject to mechanical processing. To ensure that no contamination has occurred, it is advisable to perform a chemical analysis of the material before and after ball milling. When a mortar and pestle are used to grind hard materials, excessive tapping on large pieces of a sample to break.
AGATE MORTAR AND PESTLE APPLICATION
The first step in preparing the sample is to examine the total bulk, reject any contaminated or suspect material and select the portion required for analysis. This latter may conveniently be done by selecting the portions for petrographic examination and for the reserve collection and crushing what is loft. In the case of coarse-grained and porphyritic rocks, a sample of not less than 10 kg should be available for crushing, and proportionately less of the fine-, even-grained rocks. At this and all subsequent stages in the sampling, crushing and grinding, an intelligent approach is necessary to ensure that the introduction of extraneous matter is kept to a minimum. Only then can the results of the chemical analysis of the prepared sample be taken to represent the chemical composition of the material collected.
The following notes are based upon the procedure used by the senior author to prepare igneous silicate and carbonate rocks for analysis. A simplified procedure is used for friable rocks such as unconsolidated sediments which can usually be fed directly to a mechanical agate mortar and pestle. All other samples are fed first to a small jaw crusher. The product is screened and any oversize material returned to the jaw- crusher, now set with the jaws giving a slightly smaller gap. The whole of the sample can be reduced in this way to pass a No 5 mesh sieve. This rock material is then riffled to give about 500 g which is sieved on a No 10 mesh sieve, the oversized material then being cram-fed to the jaw crusher on its finest setting.
The product is riffled once more to give 75 to 100 g of material, all of which is subsequently ground to give the sample for analysis. The grinding is done by feeding small quantities at a time to a mechanical agate mortar and pestle. The grinding is stopped from time to time to remove the 100-mesh material by sieving through bolting cloth.
Once the grinding is complete, the sample material is transferred to a large bottle – an 8 oz bottle is a convenient size – and is thoroughly mixed by shaking and rolling. After this the material is transferred to a smaller bottle and labelled with details of the specimen, locality from which it was taken, serial or catalogue number and the notebook reference. The details recorded in the notebook should include notes on the sampling, crushing and grinding procedures, sieving operations, weight of the prepared material and the date. The sample is then ready for analysis.
The practice of coning and quartering is not recommended for the sampling of the small amounts of material collected for rock analysis. A series of riffles of varying sizes can be kept for this purpose, or alternatively a rotary sampling machine can be used. Samples produced by grinding in a mechanical agate mortar and pestle are similar to those ground by hand in that they contain a great deal of fine material.
Anyone who has attempted to reduce specimens of mica minerals or rock samples containing large amounts of mica, will have experienced the difficulty of reducing platy minerals to an impalpable powder. Where mechanical mortars are used, the harder, more brittle minerals are preferentially ground, leaving the mica minerals to enrich the latter fractions. Care must be taken to ensure that none of the mica fraction is lost or discarded, and the powdered sample is thoroughly mixed before portions are taken from it. Abbey and Maxwell have reported that pre-ignition of mica samples makes the grinding stage easier, but that the ignited product slowly gains weight, making accurate weighing virtually impossible. These authors recommend the use of a “blender” with blades rotating at 15,000 rpm for size reduction of mica.
Contamination
Agate mortars and pestles are a frequent source of contamination, but introduction of extraneous material can occur at all stages in the preparation of the sample. Contamination from painted labels has already been noted. If such labels have been used, they should be removed by chipping or grinding before the sample is crushed. Jaw crushers must be cleaned particularly carefully and thoroughly if cross contamination is to be avoided. If the crusher is fitted with jaws of mild steel, small fragments may be short from the faces giving appreciable errors in the determination of ferrous iron. The amounts of tramp iron introduced in this way can be considerably reduced by fitting jaws of hardened manganese steel, but this may in turn introduce small amounts of other elements such as chromium into the sample. The practice of using iron jaws to crush the sample, followed by the removal of the introduced iron fragments with a magnet, is not to be recommended, as any magnetite present in the rock will be similarly removed together with smaller quantities of other iron minerals such as pyrrhotite and ilmenite. This would materially affect the composition of some samples, carbonatites for example.
The use of nylon bolting cloth supported in a ring of plastic material cut from an acrylic pipe, can eliminate metallic contamination at the sieving stages, but care must be taken that loss by dusting is kept to the minimum.
If many silicate analyses are to be made it is preferable to reserve a special agate mortar for grinding these samples. If ore minerals are ground in it, traces of these minerals can usually be found in subsequent samples no matter how carefully the cleaning is done.
The introduction of extraneous material is not the only change occurring in rock samples during the grinding. Water may be lost, or in some cases gained, whilst both ferrous iron and sulphur may undergo partial oxidation. These effects are enhanced by excessive grinding. For this reason we recommend that silicate rocks should be reduced in size only to pass a 70-mesh sieve. This may reduce oxidation changes, but at the same time increases the difficulty of decomposing the rock. Oxidation during grinding was clearly shown and reported that a sample of diabase (dolerite), I3 containing approximately 10 per cent FeO, gave a progressively lower FeO content as the grinding period was prolonged.
After 20 minutes the FeO content had decreased by more than 0.5 per cent and this rate of oxidation was maintained throughout the grinding period. Grinding of the rock material in the same agate mortar for 10 minutes but with continuous moistening with acetone produced a sufficiently fine material with no detectable oxidation.