- To participate in the 911Metallurgist Forums, be sure to JOIN & LOGIN
- Use Add New Topic to ask a New Question/Discussion about Hydrometallurgy.
- OR Select a Topic that Interests you.
- Use Add Reply = to Reply/Participate in a Topic/Discussion (most frequent).
Using Add Reply allows you to Attach Images or PDF files and provide a more complete input. - Use Add Comment = to comment on someone else’s Reply in an already active Topic/Discussion.
Gold dissolution decreasing with increasing cyanide concentration above the optimum value (5 replies and 1 comment)
are you maintaining oxygen concentration while increasing cyanide concentration? Could have large amounts of cyanide being chewed up by the sulphides. try getting to desired oxygen concentration in solution and staying there for several hours (pre-aeration) before adding cyanide to see if the unreacted surface sulphides are consuming cyanide
Hello, maybe you can advise me. I am grinding fine using sod. cyan. Since I don't have a vat, I am using 5 gallon buckets, mixed and forced air. I started with 200 gram cyan. for 20 lbs ground fine ore. After 2 days Strain off solution and reuse on fresh 20 lbs cons. Doing this over and over adding 15 gm. cyan. each time. For 5 times. Then going to filter and use zinc to precip.. 3 question's, is this going to work to build up gold in the cyan.? Can I rinse the cons with water and add to solution? A I adding to much cyan.? I know I have a lot of sulfur and arsenic. Thanks
dfelsher,thank you for your nice and helpful comment. In fact the experiment was done while varying oxygen concentration also. At 2 ,4 ,6 and 24 hours i checked if the amount of dissolved oxygen is still the one i started with let's say 10 ppm. In case there was a drop, i added a certain amount of hydrogen peroxide to increase dissolved oxygen to the value i started with. Please find the attached screen shot for the way the experiments were run and how these factors where varied for the three experiments.
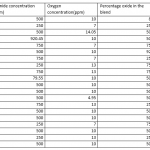
By simple thinking, this is my opinion. You concluded that there was an optimum cyanide concentration for good gold recovery rate. Maybe since you increase your cyanide concentration the other base metal from the ore has been dissolve with the solution and hindering for absorption of Activated carbon. So my suggestion regarding this situation you can add more activated carbon or after activated carbon absorption you can do zinc cemention in the solution.
Hi Jackson.
- I realised from the table as you increase cyanide, you drop the oxygen which is giving you a lower recovery. All the low recoveres are resulting from low oxyen or low oxide%. The high oxygen and high oxide CONTENT that resulted in a lower recovery had a low cyanide consumption. Let' keep the oxide CONTENT and oxygen same possibly 10ppm and vary only the cyanide from 500 and above to see the recoveries.
Maybe someone can advise me. I have never done any mining. I was willed land with 3 very old mines. Had stuff assayed, yes gold. Ok I tried smelt, acids now going to try cyanide. I dont understand all the letters-numbers. Can anyone point me to a step by step with amounts for using cy. Plan is to use zinc as precip. There is arsenic and sulfur. It has prevented acid drops w/ SMB or oxalic, but drops with lye[sod. hydrox]. Assay flux/lead works but not cost effective.
Please join and login to participate and leave a comment.

Hello!! hope everyone is OK!!!
well, going straight to my question;i was doing bottle rolling so as to establish some of the factors that will be optimum to the newly planned to process low grade sulfide gold ore gold processing plant. While doing the experiments, i varied oxygen dosage from 7 ppm, 10 ppm to 13 ppm and cyanide concentration at 250 ppm , 500 ppm and 750 ppm.Since the ore is sulfide in i also considered blending oxide with sulfide ores.These experiments were done with sulfide and oxide ore blending of 25% ,50% and 75% oxide ore. So that is to say 3 factors were varied namely: cyanide concentration, oxygen concentration and oxide-sulfide blend. In order to avoid bias,reduce the number of experiments and to reduce some error is used design of experiment using response surface methodology(in mini-tab)
However as after i have finished plotting my graphs during analysis, it has appeared that cyanide trend is different from what i previously knew. In fact gold dissolution increased as cyanide concentration increased to a maximum of around 600 ppm. However above that , gold dissolution efficiency started to decrease. This trend has left me with a question of what is the reason behind this decrease?.
Can someone help me explain if this is possible.And if its possible, what is the reason behind? I have attached a picture for reference(abnormal cyanide concentration).Please fill free to ask for more clarification if something is ambiguous about my post.
Thank you for your time
Regards,
Jackson