Table of Contents
- Solubility of Metal Xanthates in Flotation Reagents
- Lead, Copper, Zinc, and Iron
- Gold and Nickel
- Effect of Surface Oxidation on Floatability
- Flotation Control by Solubility of Metal Xanthates
- Flotation of PB-CU-FE-ZN Ore
- Flotation of CU-FE Ore:
- Climatic Conditions affect Flotation Rate
- Detailed Study of Sudbury Ore
- Description of Ore
- Preparation of Test Samples
- Flotation Machine
- Chemical Analysis
- Preliminary Test Work
- Reagents Used
- Description of Tests
- Calculated Estimate Indicated by Test Work
- Resume of Results of Tests
- Gold-Pyrite Separation
- Resume
- Conclusions
It is generally conceded by flotation men that the condition at the surface of a particle of mineral or gangue is the most important factor controlling its behaviour in a flotation cell. Much research work has been devoted to the study of the forces that are active at the surface of the particle and of the effect of the surrounding solution upon these forces.
Study of the relationship between mineral or gangue particles and gas bubbles indicates that, in the main at least, flotation is a physical phenomenon. The important factor, that of mineral-gas-bubble attachment, is unquestionably physical; however, modifications may be effected by chemical means, whereby the nature either of the surface of the particle or of the surrounding solution may be changed. For example, the coating of lead sulphide that forms on lead-carbonate ore particles when sodium sulphide is added greatly aids the flotation of the carbonate particles.
Without going into the question as to why, under certain surface conditions, mineral particles will attach themselves to gas bubbles whereas under other conditions they will remain unaffected, it may be stated that there are three general conditions in a flotation pulp which control the attachment of the particle and the gas bubble. These conditions are:
- Conditions favouring the precipitation of a water-repellent coat on or immediately surrounding the surface of a mineral particle, making it floatable. Example: a metal xanthate or sulphide precipitate.
- Conditions favouring the precipitation of a water-avid coat on or immediately surrounding the surface of a mineral particle, making it non-floatable. Example: a carbonate, hydrate, ferrocyanide, cyanide, chromate, etc.
- Conditions favouring the solution of either a water-repellent or a water-avid coat on or immediately surrounding the mineral particle which neither retards nor accelerates the natural flotation rate. Example: sulphuric acid on pyrite-chalcopyrite or sphalerite.
The three conditions stated above will apply differently to each of the several kinds of mineral particles present in the pulp because of the different chemical behaviour of the metals contained in the minerals considered.
When condition (1) applies to one mineral in the pulp and condition (2) applies to all the others that are present with it, a clean separation of this one mineral will be possible; but when condition (3) applies to some of them, they will hamper the clean separation. It is therefore desirable to have the first two conditions active to as great a degree as possible.
The accompanying curves (Figures 1 to 7), based on work carried out by the writer and D. C. Derringer in 1924-25, will serve to illustrate these points more clearly. They show, progressively, the amount of precipitation that
takes place in molecular solutions of the commonly occurring metals by addition of increasing amounts of various precipitating agents.
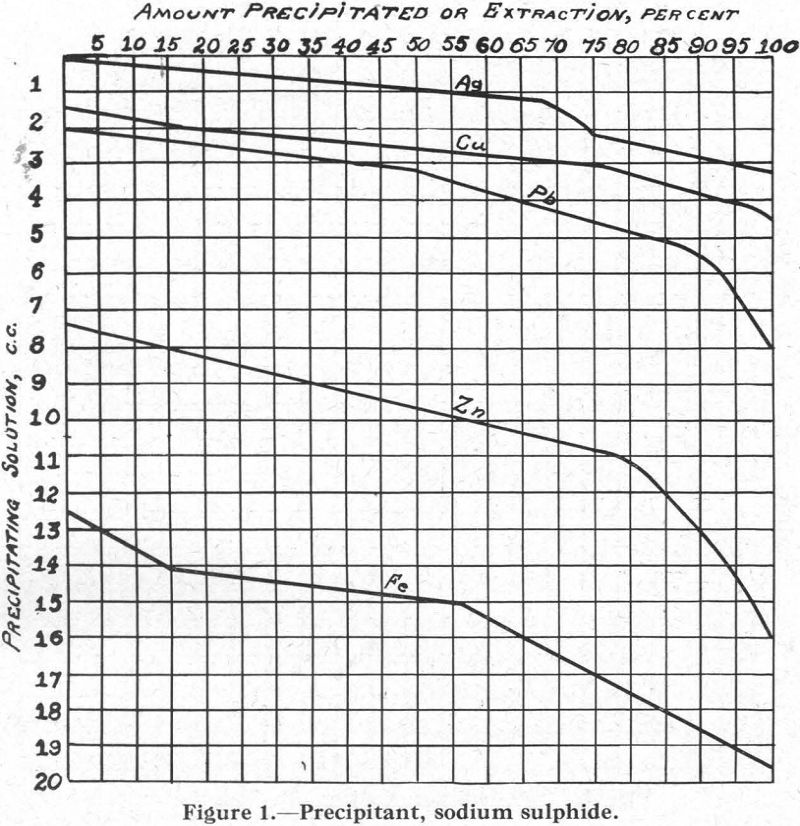
Figure 1.—Precipitant, sodium sulphide. As will be noted, the curves for the several metals follow closely Schuerman’s series of sulphur affinities.
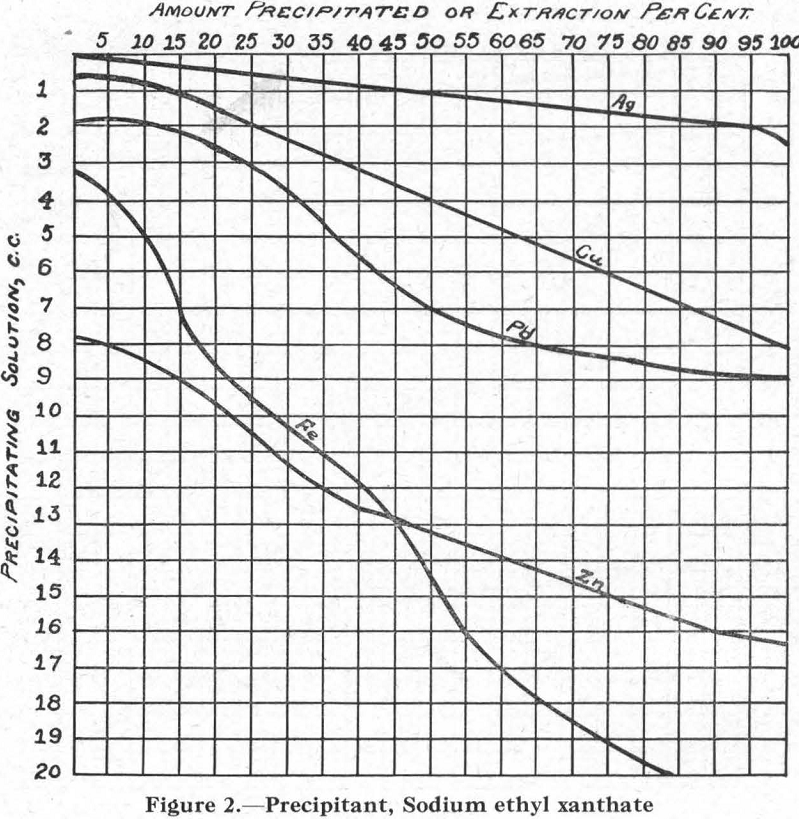
Figure 2.—Precipitant, sodium ethyl xanthate. The curves for the several metals conform nearly enough to those for all xanthates, for purposes of this discussion.
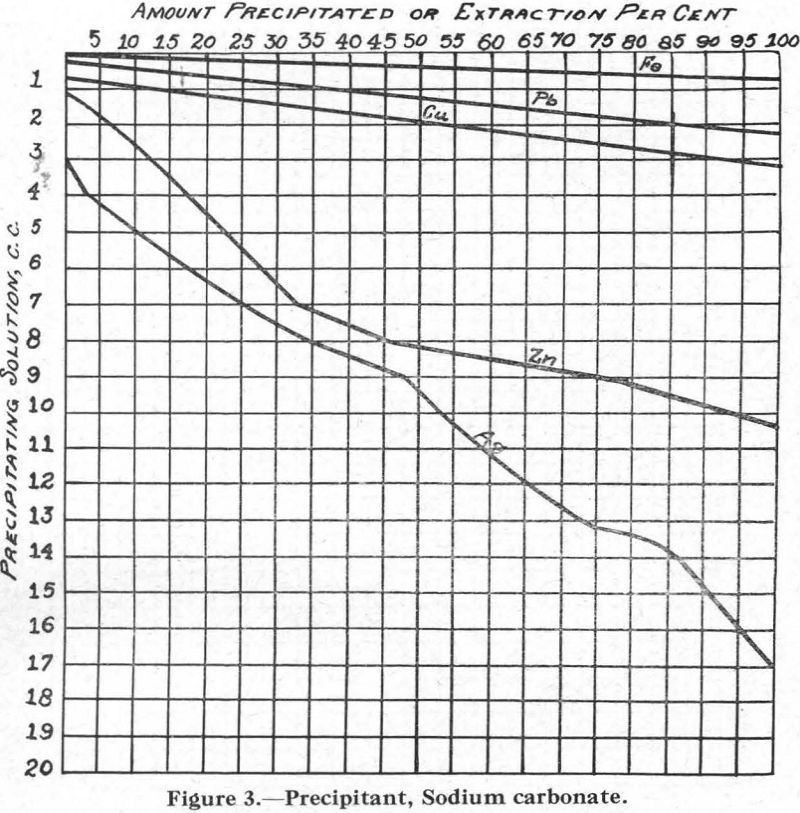
Figure 3.—Precipitant, sodium carbonate. It may be noted that lime was found to give results similar to those obtained with sodium carbonate.
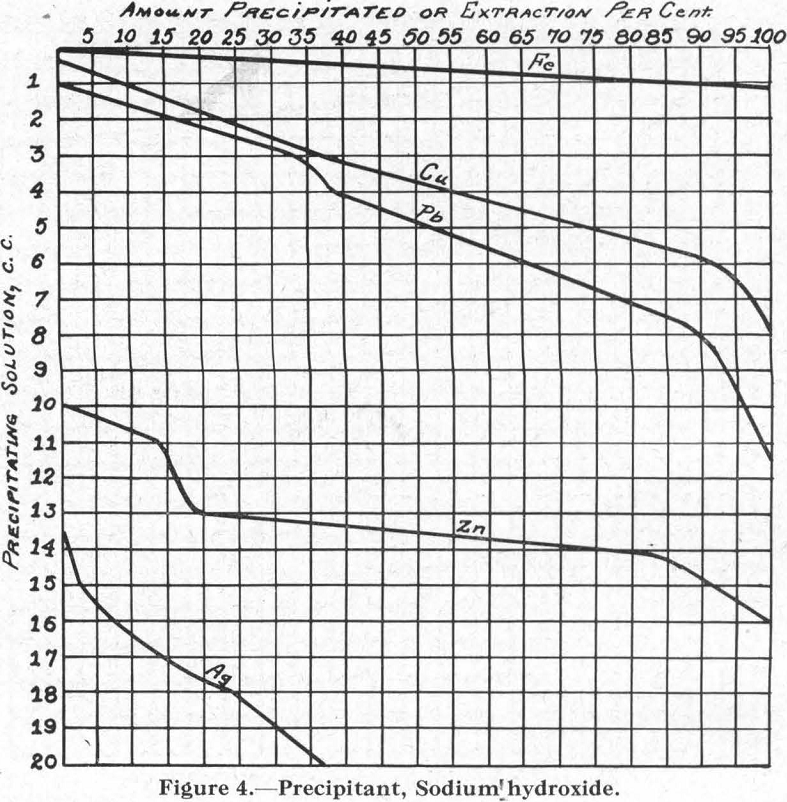
Figure 4.—Precipitant, sodium hydroxide. Comparing this with Figure 3 (for sodium carbonate), it is of interest to note the reversed positions of the curves for Pb and Cu.
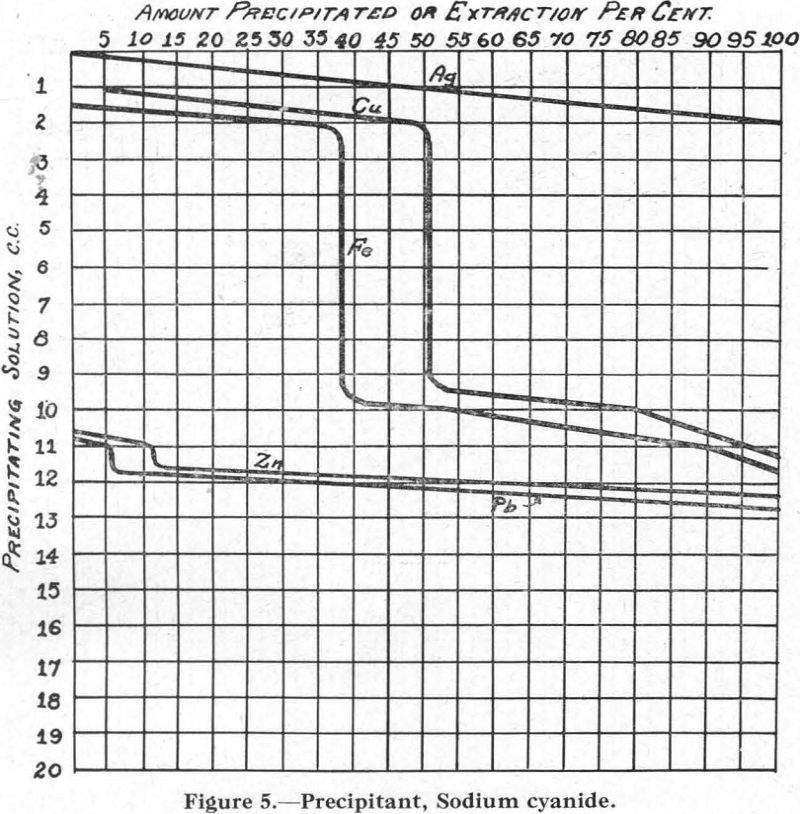
Figure 5.—Precipitant, sodium cyanide. The curves for Cu and Fe are peculiar in that, between certain points, no precipitation takes place with further addition of the sodium cyanide. This was checked several times.
When only one of these metals is present, however, its precipitation is continuous as more and more of the precipitant is added.
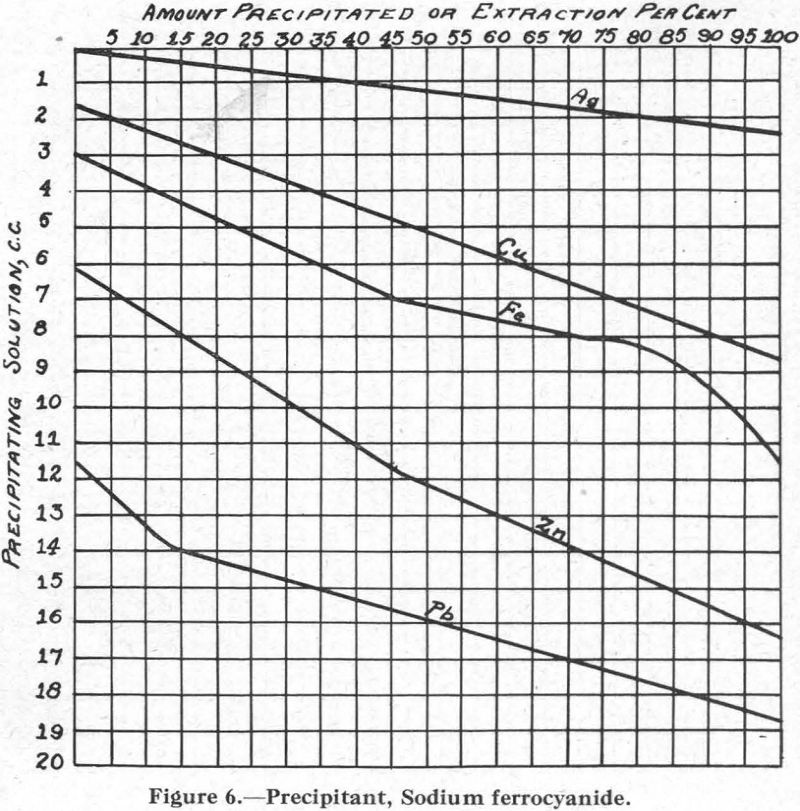
Figure 6.—Precipitant, sodium ferrocyanide. Although not in common use as a flotation reagent, sodium ferrocyanide most likely forms in most flotation circuits where soluble iron salts are present. For this reason, the curves are of interest.
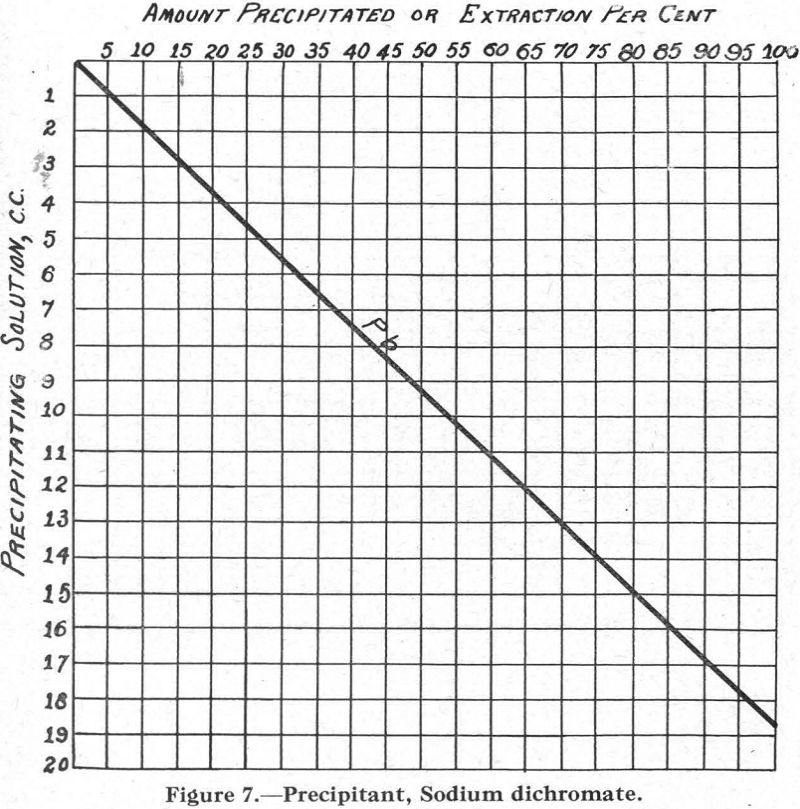
Figure 7.—Precipitant, sodium dichromate. This curve is interesting because, although the lead is the only metal precipitated, other compounds must have formed in the solution to slow up the precipitation of the lead as chromate.
For a discussion of chemical reactions that may take place in a flotation pulp reference may be made to the very comprehensive paper by Taggart, Taylor, and Knoll entitled Chemical Reactions in Flotation (Publication No. 312, A.I.M.E., Vol. B30, Transactions, Milling Methods, 1930).
The present paper will be restricted, first, to a consideration of the effect that the relative solubilities of various metal xanthates have on their floata-
bility in solutions of the reagents ordinarily used in flotation practice at the present time, and second, to the outlining of a differential flotation scheme based on the results of the determination of the solubilities of certain metal xanthates not commonly used in practice, and followed by actual flotation test work. Sulphide minerals only will be dealt with. The object of the paper is to show that the explanation of differential flotation, using a soluble thiocarbonate such as amyl xanthate, can be clarified by considering the solubilities of the metal xanthates. Present flotation practice in galena-chalcopyrite-sphalerite-pyrite separations, as explained by the relative solubilities of the xanthates of Pb, Cu, Zn, and Fe, was used as a guide.to a flotation separation of pentlandite-chalcopyrite-pyrrhotite by commencing with a study of the relative solubilities of the xanthates of Ni, Cu, and Fe.
The paper also includes a consideration of the relative solubilities of the xanthates of Au, Cu, and Fe and outlines a method for the separation of gold and chalcopyrite from pyrite.
Solubility of Metal Xanthates in Flotation Reagents
The absolute solubilities of the metal xanthates in the several reagents used in flotation practice were not investigated. As relative solubilities are what are encountered in practice, they are considered sufficiently indicative.
Lead, Copper, Zinc, and Iron
First of all, the relative solubilities of the xanthates of the several metals to be considered (Pb, Cu, Zn, and Fe) were determined. A solution was made containing molecular equivalents of the nitrates of these metals in distilled water. This solution was divided into ten equal parts, each in a 400 c.c. beaker, and the amount of freshly made pentosal amyl xanthate necessary to precipitate all the metals in one beaker was determined. Then, to the solution in the first beaker was added 10 per cent of the total amount of xanthate necessary to precipitate all metals present. In each succeeding
beaker the amount of xanthate added was increased 10 per cent, until, in the tenth beaker, sufficient was added to precipitate all the metals present.
At first, some difficulty was encountered owing to the fact that, in filtering, the fine precipitates tended to pass through the filter paper, but it was found that boiling aided in coagulation of the precipitate and did not interfere with the results.
The relative solubilities of the several metal xanthates are indicated at the extreme left (column I) of Figure 8, in which solubilities are arranged in increasing order from top (6) to bottom (1). For the four metals Cu, Pb, Zn, and Fe, copper xanthate is the least soluble, followed in order by the xanthates of Pb, Zn, and Fe, the most soluble (see also Figure 2).
The relative solubility of these metal xanthates in presence of various other reagents was then studied. The results are shown in Figure 8.
Column II.—Xanthate and cyanide. A solution of the nitrates of the metals was prepared and divided into ten equal parts as before. To each was added
sufficient sodium carbonate to give a pH of from 7.2 to 7.5, and then enough pentosal amyl xanthate to precipitate all the metals present. In practice, the addition of 0.2 lb. xanthate per ton of ore will usually effect an efficient recovery of a mineral from an ore pulp; also, the retardation effect of 0.2 lb. of sodium cyanide per ton is usually ample to aid the separation of, say, galena from chalcopyrite and sphalerite. This equivalence in amount of xanthate and cyanide, as used in practice, was made the basis for the amount of cyanide to be used in the laboratory experiments. To the first beaker, then, was added one-fourth as much cyanide as it contained xanthate, and regularly increasing quantities were added to each of the succeeding beakers until, in the last (tenth) one the amount of cyanide added was equal to twice the amount of xanthate it contained.
Under these conditions, it was found that zinc xanthate is the most soluble of the four present, and the others follow in the order Cu, Fe, Pb (Column 2, Figure 8). The lead xanthate was practically insoluble. The iron showed its presence as a precipitate throughout, but presumably as a combination cyanide-hydrate and not as xanthate.
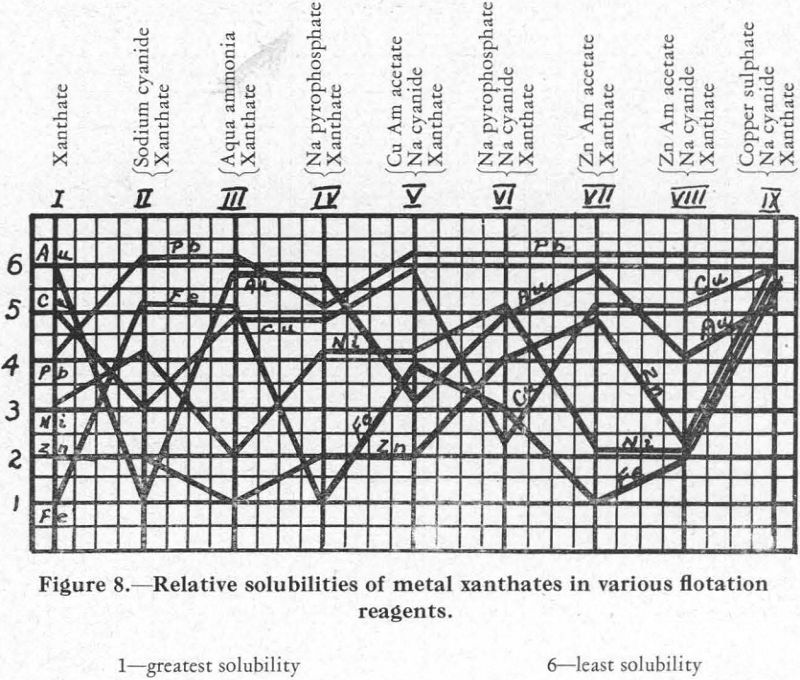
Column III.—Xanthate and aqua ammonia. The lead, copper, and iron are insoluble (the iron as a hydrate) and the zinc is soluble.
Column V.—Xanthate, cyanide, and copper ammonium acetate. In this experiment cyanide and xanthate equivalent to 0.2 lb. per ton were added in each of the ten beakers, and then varying amounts of a solution of copper ammonium acetate. Result: lead and copper (as xanthate) and iron (as cyanide-hydrate) precipitated; zinc in solution. This series showed clearly the effect of the copper ammonium acetate on the copper xanthate and the zinc xanthate. Addition of this reagent allowed zinc xanthate to go into solution, presumably in the ammonia set free by the reaction between copper ammonium acetate and cyanide, and the excess copper formed a complex copper-cyanide ion which held up the CN ions and allowed the precipitation of the copper xanthate.
Column IX.—Xanthate, cyanide, and copper sulphate. Procedure similar to the last. Result: all metal salts precipitated as soon as sufficient copper ion was present to hold the cyanide radical.
Resume.—These experiments indicate that, on addition of sodium cyanide to a solution, the metal salts of which have all been precipitated as xanthates, the precipitate of lead xanthate will be insoluble and a precipitate will be formed of the iron as a cyanide-hydrate, while copper and zinc will go into solution as cyanides. Copper ammonium acetate added to the filtered solution?will completely precipitate the copper as xanthate and the remaining iron as hydrate. The filtrate from this will contain zinc xanthate, still soluble because of the presence of cyanide and ammonia ions—probably because of the ammonia ions principally. Addition of copper sulphate to this filtrate will precipitate the zinc as a zinc-copper xanthate, and will also throw down a certain amount of copper cyanide, because the copper ion will be in excess.
The metals investigated in the experiments outlined above occur in ores that are actually being treated by flotation in a number of mills and, later in this paper, they are considered from that standpoint. Among other metals which are not so common are nickel and gold. The behaviour of these was next studied.
Gold and Nickel
A solution was made up containing molecular equivalents of the metals Ni, Au, Cu and Fe as chlorides and the investigation of this proceeded along the same lines as outlined for the Cu-Pb-Fe-Zn nitrate solution. Some difficulty was encountered with the iron as it hydrolized and precipitated as hydroxide as soon as the solution was made just alkaline. This, however, did not affect all the results, because the relationship of the nickel and gold with the copper gave the desired information. The results of these experiments are included with the others in Figure 8.
Column I.—Xanthate alone. The gold precipitated first, followed by the copper, nickel, and iron in the order named. A subsequent experiment showed that nickel comes down between lead and zinc.
Column II.—Xanthate and cyanide. Gold xanthate is the most soluble, and nickel xanthate is just a little less soluble than copper xanthate.
Column III .—Xanthate and aqua ammonia. The gold xanthate remained quite insoluble, together with the copper, lead, and iron. The nickel xanth¬ate went into partial solution with the zinc xanthate.
Column V.—Xanthate, cyanide, and copper ammonium acetate. Addition of the acetate counteracted the solubility of nickel xanthate in cyanide solu¬tion but only partially affected the gold xanthate.
Column IX.—Xanthate, cyanide, and copper sulphate. The sulphate aided the complete precipitation of both the gold and nickel. However, a small part of the gold was soluble, indicating a solubility in a metal cyanide.
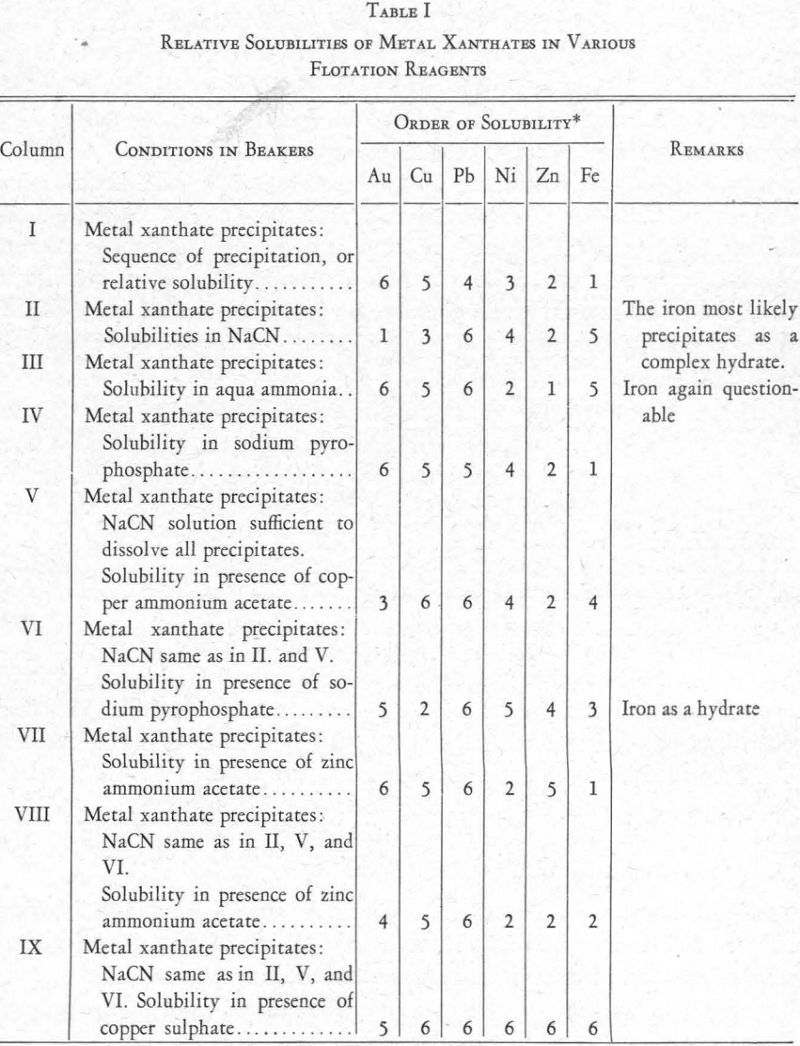
Column IV.—Xanthate and sodium pyrophosphate. In addition to the reagents already referred to, a number of others were tried, with varying results. Sodium pyrophosphate, especially, seemed to offer possibilities. Prescott and Johnson, in their Qualitative Analysis, say: “A solution of potassium xanthate precipitates neutral solutions of nickel and cobalt, the former being soluble in ammonium hydroxide. The xanthate also precipitates nickel in alkaline solutions in presence of sodium pyrophosphate (separation from Fe)”. Using the same procedure as in the tests already described, it was found that sodium pyrophosphate prevented the formation of iron xanthate but allowed the precipitation of Au, Ni, and Cu as xanthates.
In a similar experiment, using a solution of the nitrates of Ni, Pb, Cu, Zn, and Fe, it was found that the xanthates of Ni, Pb, and Cu are insoluble when sodium pyrophosphate is present.
Column VI.—Xanthate, cyanide, and sodium pyrophosphate. Cyanide in addition to sodium pyrophosphate put the copper and gold into solution, but the nickel xanthate formed and the iron precipitated incompletely, presumably as ferric hydrate.
Column VII.—Xanthate and zinc ammonium acetate. The acetate showed a tendency to put nickel and iron xanthates into solution. This is because of the solubility of nickel xanthate in aqua ammonia and of iron xanthate in acetic acid.
Column VIII.—Xanthate, cyanide, and zinc ammonium acetate. The presence of cyanide with zinc ammonium acetate changed conditions with respect to the gold and zinc xanthates, increasing the solubility of both.
The foregoing data relating to the solubility of metal xanthates in various flotation reagents are summarized in Figure 8 and also in Table I. In each, the numeral 1 indicates greatest solubility and 6 least solubility.
Effect of Surface Oxidation on Floatability
As the purpose of this paper is to show that these chemical data can be used to explain the behaviour of certain sulphides in a flotation cell, this matter will now be considered. It is essential at the outset to bear in mind the fact that a film of a soluble metal salt is continuously forming on the surface of these sulphides. This matter is very fully discussed in the paper by Taggart, Taylor, and Knoll, already referred to, which contains a mass of data in support of the claim that sulphide minerals are soluble in water.
The statement that sulphide minerals are soluble in water should be expanded to include the salts formed by their oxidation, for, as the writer assumes, it is these oxidized salts that are present in greatest concentration in the solution or envelope immediately surrounding the sulphide particles, when they are in a pulp in a flotation circuit.
This surface oxidation is a necessary part of flotation mechanics. As the writer sees it, there are two-forms of oxidation that must be given consideration in flotation:
- Oxidation occurring at the surface of a mineral particle, while it is in the ground, in a stope, in a dump, in transit to the mill, or in the mill-bin; that is, oxidation in a minimum of water and a maximum of air.
- Oxidation occurring at the surface of a mineral particle while in the mill-pulp; that is, oxidation in a maximum of water and a minimum of air.
The first type of oxidation, that in a maximum of air, is detrimental to flotation because it usually leaves the oxidation product on the surface of the mineral particle, and although this oxidation product may, at the outset, have been a soluble thiosulphate, it eventually is changed to an insoluble compound, usually an oxide of the metal contained in the mineral.
The second type of oxidation, that in a maximum of water, is helpful to flotation because it furnishes a salt with which the various reagents react to give flotation control. Oxidizers such as ozone and atomic oxygen, produced in the electrolysis of water, can be considered the reagents that react with the soluble salt first formed, converting it to the oxide of the metal so rapidly that the surrounding solution cannot carry it away. This leaves an oxide of the metal, possibly on the surface of the mineral particle, which retards the flotation action in a manner similar to the oxidation that occurs in a maximum of air.
The soluble metal salts resulting from the oxidation of the sulphide minerals are considered to be essentially similar, in their effect, to the metal solutions that were used in the chemical work already described, with the exception of those of gold. Gold is only slightly soluble in solutions of ferric and cupric salts (Comey and Hahn, Dictionary of Chemical Solubilities). Its solubility may be increased by certain reagents and modified by others. This subject is discussed on a later page.
These soluble metal salts are susceptible to reactions with the solutions of the flotation reagents that are in contact with or immediately surrounding the mineral particle. Reference is made to the space immediately surrounding the mineral particle because there must be room somewhere for outside ions, principally of oxygen, to come in contact with the metal and sulphur ions of the mineral in order to form something for the flotation reagent to react with. The precipitate or solution resulting from the reaction between the soluble metal salts derived from the mineral in question and the flotation reagents present, will be the controlling force in the flotation action to follow.
Flotation Control by Solubility of Metal Xanthates
Concisely stated, the writer’s conception of flotation control by the solubility of the metal xanthates is as follows:
- Chemical conditions in a concentrating mill pulp which favour the precipitation of the xanthate of a metal contained in a mineral, will cause the mineral in question to become attached to the gas bubble more firmly than the original sulphide mineral, and consequently will accelerate its flotation rate.
- Chemical conditions in a concentrating mill pulp which favour the solution of the xanthate of a metal contained in a mineral will prevent the acceleration of the natural flotation rate of the original sulphide mineral (1) and allow room on the surface of this mineral for other chemical reactions which will retard the flotation rate of the original sulphide mineral.
Practical experience, also, has convinced the writer that a mineral particle which is coated with the xanthate of one of its constituent metals, or which is immersed in a solution that favours the precipitation of the metal xanthate in question, is more resistant to wetting by water than the original sulphide under the same conditions of reagents in solution. It will therefore be more firmly attached to the gas bubble and consequently will have a more rapid flotation rate than the original sulphide.
In a certain mill for whose design and flow-sheet the writer was responsible, it was necessary to remove 25 to 40 tons a day—or from 10 per cent to 15 per cent of the total tonnage—as a talc froth prior to the extraction of the metal sulphides. At times, as high as one pound of cresylic acid per ton of pulp was necessary in order to remove this material, but the loss due to metal sulphides floating with this talc was less than 5 per cent. After the removal of the talc froth, xanthate introduced into the pulp immediately replaced the remaining talc particles in the bubbles with galena and chalcopyrite particles, without additional cresylic acid, showing conclusively that the xanthate-prepared surface or surroundings of the particles of galena and chalcopyrite were less water-avid or more water-repellent than the original sulphide.
Flotation of PB-CU-FE-ZN Ore
Practical application of the xanthate solubility hypothesis outlined in the foregoing pages may be illustrated by the flow-sheets of two differential flotation plants with which the writer is familiar. One of these plants produced a lead concentrate, a copper concentrate, and a zinc concentrate. Average results obtained were about as follows:
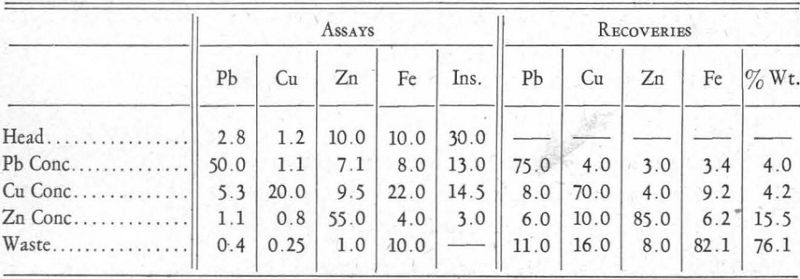
This ore was very finely disseminated, requiring extremely fine grinding, and it also contained a large amount of talc material which hindered operations, as is evident from the high percentage of insoluble in the lead and copper concentrates. The following flotation reagents were used:
Before talc froth: soda ash, cyanide, zinc sulphate, cresylic acid and pine oil.
Before lead froth: xanthate.
Before copper froth: copper ammonium acetate, cyanide, xanthate.
Before zinc froth: copper sulphate, xanthate, pine oil.
In connection with the description that follows, reference should be made to the solubility curves, Figures 1 to 8, and to Table I.
Talc Flotation.—The soda ash and cyanide can be considered to have slighly hindered the flotation rate of the metal sulphides by favouring the formation of carbonates and cyanides, as indicated in Figures 3 and 5, thus preventing their flotation in the presence of frothing agents such as creslyic acid and pine oil. Result: flotation of talc.
Galena Flotation.—The introduction of xanthate made possible the formation of metal xanthates. Those of copper and zinc, being soluble in cyanide, could not form (Figure 8). Iron xanthate did not form at all, because of its great solubility in water and its still greater solubility in cyanide, and also because of the insolubility of the carbonate (Figure 3) or cyanide-hydrate formed before the introduction of the xanthate. The xanthate of lead formed because conditions are right for its formation, it being practically insoluble in water or in cyanide solution. Result: flotation of galena.
Resume:
(a) Lead xanthate; insoluble in water or cyanide.
(b) Copper xanthate; soluble in cyanide solution.
(c) Zinc xanthate; soluble in cyanide solution.
(d) Iron xanthate; probably not formed because of its solubility, and because of the insolubility of a former product of reaction, iron carbonate or cyanide-hydrate.
Chalcopyrite Flotation.—Copper ammonium acetate combines with cyanide to form an insoluble complex copper-cyanide ion which allows the precipitation of the copper xanthate, at the same time releasing ammonia ions which|hold in solution zinc xanthate. Result: flotation of chalcopyrite.
Resume:
(a) Copper xanthate; insoluble in absence of cyanide.
(b) Zinc xanthate; soluble in ammonia and ammonium compounds.
(c) Iron xanthate; same as in first froth.
Sphalerite Flotation.—Copper sulphate forms copper ammoniate, which is an undissociated colloidal product, allowing the formation of zinc xanthate or possibly a double copper-zinc xanthate. In sufficient quantities, copper sulphate will react with the coated iron mineral, provided it has satisfied the soluble alkaline reagents in solution, allowing the formation of probably a double copper-iron xanthate. Result: flotation of sphalerite.
Resume:
(a) Zinc xanthate, or a double zinc-copper xanthate, insoluble in absence of ammonia and ammonium compounds. (b) Iron xanthate; same as in first froth.
Flotation of CU-FE Ore:
The other differential flotation plant referred to produces a copper concentrate and an iron concentrate. A day’s run taken at random would average as follows:
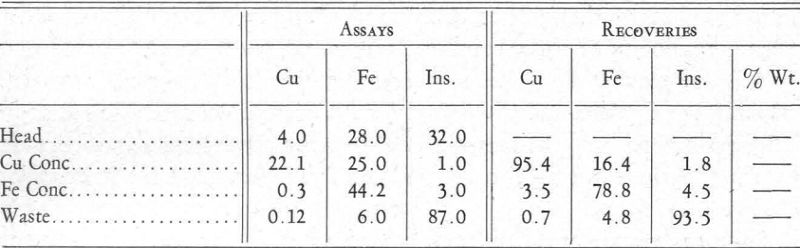
Reagents used were:
Before chalcopyrite flotation: soda ash, sodium cyanide, copper ammonium acetate, xanthate, pine oil and/or cresylic acid.
Before pyrite flotation: copper sulphate, xanthate, pine oil.
Chalcopyrite Flotation.—The soda ash was introduced first, then the cyanide, and lastly the copper ammonium acetate. The soda ash hindered the flotation of the iron minerals by favouring the formation of a carbonate. This water-avid coat on the pyrite was aided by the action of the cyanide. Also, the cyanide prevented the formation of copper xanthate because this salt is soluble in cyanide. The introduction of copper ammonium acetate formed a complex copper-cyanide ion and allowed the precipitation of copper xanthate. The ammonia ions that were set free neither aided nor hindered the formation of iron xanthate. Result: flotation of the copper minerals away from the iron minerals and gangue.
Resume:
(a) Copper xanthate; insoluble in absence of active cyanide ion.
(b) Iron xanthate; probably not formed because of its solubility, and because of the insolubility of a former product of reaction, the iron carbonate or cyanide-hydrate.
Pyrite Flotation.—Copper sulphate in neutral or slightly acid circuit forces the formation of a copper, or possibly a double copper-iron, xanthate with the iron mineral that had been coated or deadened in the first operation. Result: flotation of pyrite.
Resume:
(a) Iron xanthate; made possible, through a probable combination with copper xanthate, because of the preponderance of the copper ions.
Climatic Conditions affect Flotation Rate
Both of the ores discussed above came from mines in Canada where the ore-bodies, from the outcrop down, consist of primary sulphides, with no secondary minerals due to oxidation in evidence. Mention is made of this because ores from southwestern United States and Mexico do not respond as well as these to the same treatment and often need the addition of other reagents to modify a surface condition caused, no doubt, by their oxidation. Thus, in one ore that the writer encountered, it was found impossible to make a separation of galena and chalcopyrite with cyanide concentration as high as five pounds per ton, the reason probably being that there was a coat on the chalcopyrite capable of forming a metal xanthate insoluble in cyanide, possibly a lead salt.
Another example of the different behaviour of similar ores from different climates was met with by the writer in two mills, one in Quebec and the other in Zacatecas. On the same grade of ore, the time needed in the flotation cells in the Zacatecas mill was four times as long as in the Quebec mill. The Quebec ore-body outcropped as a sulphide; that in Zacatecas had been mined as an oxide for nine hundred feet below the surface, where sulphides were encountered. This condition in the latter case made possible a coating on the ore particles, hindering the chemical action necessary to make the flotation action clean-cut and rapid.
Another important point which must be given consideration is the soluble-salt content in mine and mill waters. In this connection, account should also be taken of the waters that were circulating in the ore-body prior to the extraction of the ore, since these may have carried soluble salts that precipitated secondary minerals on the surfaces of the primary ore minerals. Particles of primary ore whose surfaces are covered with secondary minerals will act, in a flotation machine, in the same way as would a particle having the composition of the secondary mineral. An example is the precipitation of copper sulphide (from a copper sulphate solution) on a particle of sphalerite or pyrite. Such coatings of secondary minerals will completely upset any chemical or physico-chemical theory for the flotation of the primary ore unless their effect is corrected prior to the introduction of the flotation reagents.
Attention is called to these points because they must be taken into account before any theories or hypotheses can be applied to practical operations. In the two cases of differential flotation that were considered above, the ores were of the type that might be expected to conform to theoretical predictions and give good results. Both were from Canadian mines in which the ore-bodies, from the grass-roots down, consist of primary ore with no trace of the slightest oxidation. The flow-sheet for one of these ores—the mixed Pb-Cu-Zn-Fe sulphide—had been worked out and had proved commercially satisfactory long before the metal xanthate solubility theory had been developed by the writer. For this reason, it does not afford such convincing evidence of the soundness of this theory as it would have done had the chemical reasoning been worked out first and then flotation practice used to confirm it.
Realizing the necessity for stronger supporting evidence, the writer decided to work on a differential flotation separation that up to that time had proved not entirely satisfactory in practice, with the object of determining whether, from a study of the chemical data involved, a practical flotation scheme could be worked out. The ore selected for this test was from the Sudbury district, and involved the separation of pentlandite, chalcopyrite, and pyrrhotite. Later, another ore was investigated, in this case a gold-pyrite separation.
Detailed Study of Sudbury Ore
At the time this work was undertaken, concentrating mills treating the Ni-Cu-Fe ores of the Sudbury district were successfully making high-grade copper concentrates but the nickel concentrates were of lower grade. Theoretically, however, there seemed no reason why it should not be possible to make a nickel concentrate equal in grade to the copper concentrate, since the predominant nickel mineral, pentlandite, contains only slightly less nickel than the predominant copper mineral, chalcopyrite, contains copper. Believing that this lack of success was due to insufficient metallurgical data concerning the separation of minerals containing copper, nickel, and iron, the writer decided to make a study of the Sudbury ore along the lines outlined in the earlier part of this paper. It was felt that any results obtained in such an investigation would be of practical as well as theoretical value.
Description of Ore
One of the mining companies operating in the Sudbury district very kindly agreed to co-operate by furnishing samples of ore for this research, which was carried out in the laboratories of the mining and metallurgical department at Queen’s University. The ore came from what is classed geologically as a marginal deposit. It was sent from the mine to the laboratory in small lots as required in order to avoid, as far as possible, any oxidation of the sulphides, which would affect flotation results. The average composition of all the ore received was as follows:
Copper…………………………………1 to 1.4 per cent
Nickel………………………………….2 to 2.8 per cent
Iron……………………………………38 to 30 per cent
Insoluble…………………………….35 to 30 per cent
Oxidation, no doubt, influenced all the results to a slight extent, as all samples worked on were dry before they reached the last stage of the pulp preparation, i.e., grinding. However, owing to the relatively slow rate of oxidation of all sulphides in Canadian ores, as evidenced by the absence of any extensive oxidized zones, the effect of this oxidation is much less than it would be in the case of ores from districts where such oxidation products are common. In this investigation no account was taken of the platinum-group metals or of gold, as these are present in the ore in extremely minute amount only.
The principal minerals in the ore were: pyrrhotite, Fe11S12; pentlandite, (Fe,Ni)S; and chalcopyrite, CuFeS2. Other minerals may have been present, but apart from the possibility of nickeliferous pyrrhotite, none were observed. The gangue, being of small importance in the work in hand, was not studied in detail.
Microscopic examination of screened ore and also of polished sections showed that it would most likely be necessary to grind to practically all through-200-mesh in order to effect a separation of the sulphides. The chalcopyrite and pyrrhotite were easily recognizable, but it was more difficult to distinguish the pentlandite. However, on polished sections this mineral seemed to assume a higher polish than the others, and, when dug out of the polished section with a needle, these highly polished particles were found to be practically non-magnetic. In the preparation of samples of the test products for microscopic analysis, the gangue material, if any, was first washed away in a gold-pan. The mineral particles, mostly chalcopyrite, pentlandite, and pyrrhotite, were then introduced into a flat dish containing alcohol and this was placed on the table of a binocular ore-dressing microscope. With the use of a magnetized needle it was then possible to separate the pentlandite and pyrrhotite, the alcohol seeming to act as a dispersing agent and preventing, to a large extent, the mechanical entrapping of any small particles of pentlandite in the pyrrhotite Which clung to the magnetic needle while it was being moved, under the alcohol, from one side of the dish to the other. The chalcopyrite, of course, remained with the pentlandite, as did any portion of the gangue which was not washed out at first; but these could be easily distinguished and their quantity estimated after the pyrrhotite had been removed.
Preparation of Test Samples
Samples were received from the mine in sacks and were usually lumps with a minimum of fines. In order to minimize the possible effect of oxidation, only about fifty pounds of ore was used at a time. Such a sample was crushed in a jaw crusher and passed twice through a small set of laboratory rolls until all would pass an 1/8 in. to 3/16 in. opening. This crushed sample was then divided with a sample splitter into 3,000-gram lots, which were sacked and placed ready for the rod-mill.
This method made each 50 pound series of 3,000 gram samples equal to a split sample, so that all tests on a series could be run with confidence in the fact that the same amount of mineral was always present.
The 3,000-gram sample was ground wet in a small laboratory rod-mill with 2,000 c.c. of water for a length of time necessary to produce the fineness of product desired, 95 per cent -200-mesh (0.074 mm.).
Flotation Machine
The flotation machine used was of the Denver Sub-A type. The spitz of the machine was filled with a mixture, in equal parts, of sand and cement, to a point six inches back from the froth-overflow lip. This left a space of from 1 in. to 1½ in. between the cement block and the partition between the spitz and agitation compartment. On the top of the cement block in the spitz a white glazed-tile was set in, flush with the froth-overflow lip and having a fall of about one inch in one foot towards the lip.
This cement block reduced the distance the froth had to travel in the spitz from the agitating compartment to the overflow lip, and the white glazed-tile top acted as a very effective plaque for visual inspection of the mineral in the froth when sprayed with a fine stream of water. Also, a test machine so altered, with its smaller spitz, simulated to a much greater degree the large machines in actual mill operation than did the machine as received from the manufacturer.
All test products were put through a pressure filter and dried at low heat on electric hot plates.
Chemical Analysis
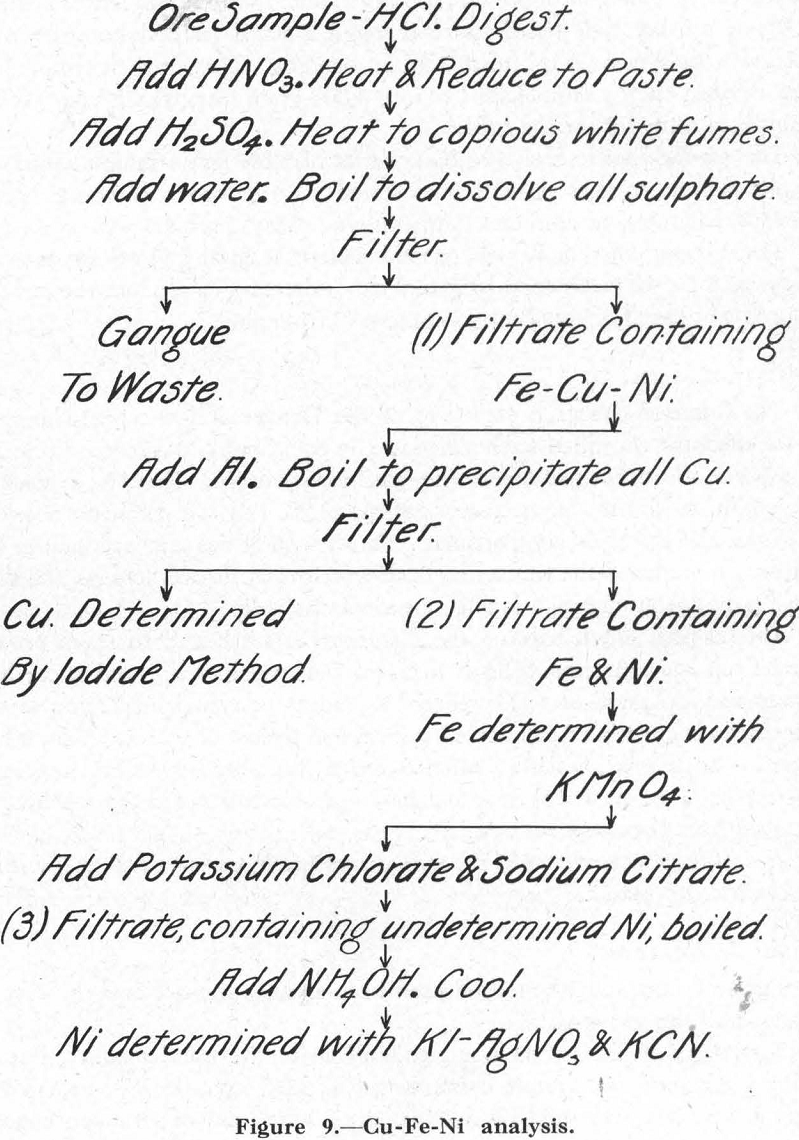
Figure 9 outlines the method used in analyzing the ore and the several products of the test work.
Titrating solutions were not standardized in the usual manner, but by using a standard ore sample containing Cu, 9.2%; Ni, 16.2%; Fe, 35.4%. This sample was prepared by first grinding a mixture of flotation concentrates all through-200-mesh, then thoroughly mixing them in a laboratory pebble-mill. The product so obtained was carefully split into samples which were sent to various laboratories for analysis. The average of these analyses was taken as representing the composition of the standard ore sample for
purposes of standardizing the titrating solutions. These were re-standardized for each new analysis. That is, every time a set of samples were to be analyzed, two samples of the standard ore were also run and the factor for each solution re-calculated. In the writer’s opinion, this procedure is particularly to be recommended for those who are not skilled chemists, and also in cases where the titrating solutions are used only once or twice a week, when, as a consequence, some solutions may remain unused for a couple of months or so.
On all concentrate products, one-gram samples were used; on middling products, from two- to three-gram samples; and on waste products such as final tailing, five grams. Spitting during the dissolving period, when large samples such as five grams were taken for analysis, was prevented by treating each sample separately over a gas flame.
This method gave very good checks of the parts against the whole except in the case of the iron, for which the total of the parts was always found to be too high. This, however, was traced to iron from the rod-mill, which could easily be distinguished with a magnet and also by the fact that it rusted quickly. However, it was not practicable to remove this iron from the samples for analysis because of the difficulty of separating pyrrhotite from metallic iron.
Preliminary Test Work
As the writer saw it, the fundamental problem in respect to these Ni-Cu- Fe ores was to produce a nickel concentrate as near the theoretical analysis of the nickel mineral, pentlandite, as possible, and at the same time maintain a commercial recovery. Also, to produce a copper concentrate with equal characteristics.
Too many metallurgists had worked on this problem to allow the writer any hope of success by following known flotation schemes.
The chemical work outlined in the early part of this paper had shown that the xanthates of Ni, Cu, and Fe are somewhat soluble in cyanide (See Figure 8), but that the introduction of copper ammonium acetate counteracts the effect of the dissolving power of cyanide and allows both the nickel and copper xanthates to precipitate, leaving the iron xanthate in solution, if it was formed prior to the precipitation of the iron, as a hydrate or complex cyanide; also that, in the presence of cyanide and zinc ammonium acetate, nickel and iron xanthates are more soluble than copper xanthate.
This suggested floating the nickel and copper minerals away from the iron minerals and gangue. This was done in alkaline circuit in the presence of cyanide and copper ammonium acetate. The nickel and copper minerals were then conditioned with zinc ammonium acetate with resultant formation of copper xanthate but not nickel xanthate, which allowed the flotation of the copper minerals.
It had been found, further, that xanthate of iron is soluble in sodium pyrophosphate but that the xanthates of copper and nickel are insoluble; and that, although the xanthates of copper and nickel are soluble in cyanide, xanthate of nickel will form in the presence of sodium pyrophosphate and cyanide.
This suggested the flotation of the copper and nickel minerals away from the iron minerals and gangue in the presence of sodium pyrophosphate and, in a separate operation, the separation of the nickel and copper minerals by the use of cyanide in the presence of sodium pyrophosphate, under which conditions copper xanthate would remain in solution while nickel xanthate would be formed, i.e., the nickel mineral would float.
Thus, the chemical data indicated that, by changing the reagents, either the pentlandite could be caused to float ahead of the chalcopyrite or, alternatively, the chalcopyrite could be floated off ahead of the pentlandite. Flotation tests, described below, showed that such is the case.
Some seventy-two tests were run on this ore. Owing to limitations in time, only those tests that seemed to be right visually were saved, and only those that it was thought would identify a trend were analyzed. The test work can be divided into three classes:
- Work with known reagents and copper ammonium acetate.
- Work with known reagents, copper ammonium acetate, and zinc compounds.
- Work with known reagents, sodium pyrophosphate, and copper ammonium acetate.
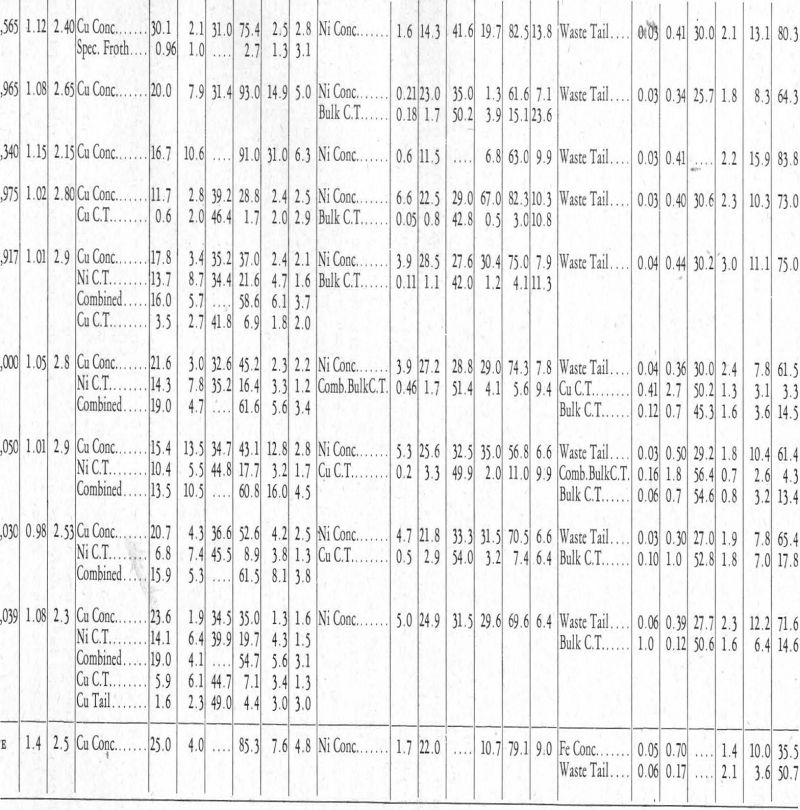
More or less detailed descriptions of the test operations follow. For a clearer understanding of these, reference should be made to Table II.
Reagents Used
Miscellaneous.—Ten per cent solutions by weight of soda ash, lime, copper sulphate, sodium cyanide, and sodium pyrophosphate were made up at sufficiently frequent intervals to avoid any danger of unsatisfactory results that might arise from the use of partially decomposed solutions.
Xanthate.—In the early work, first ethyl xanthate, and, later, amyl xanthate, as received from the dealers was used. Eventually, however, these were discarded in favour of iso-amyl xanthate, which was made up at least weekly from iso-amyl alcohol, carbon disulphide, and potassium hydroxide.
Copper Ammonium Acetate (C.A.A.).—For test work, in order to have a standard, a solution was made by placing an excess of copper oxide, procured from the ammonia leach plant of the Calumet and Hecla Company, in a one-litre bottle, adding 100 grams of ammonium acetate and 100 c.c. of aqua ammonia, and making up to one litre. This gave a solution containing about 0.5 per cent Cu, or 2 per cent Cu(NH3)4(C2H3O2)2.
In practice, the procedure followed was to pass compressed anhydrous ammonia gas through a weak solution of acetic acid until the solution turned litmus distinctly blue. In this solution was placed, in a porous bag, an excess of copper oxide, which was allowed to remain immersed for 36 hours, or until the resulting solution contained from 0.8 per cent to 1.0 per cent Cu. This solution must be made up in wooden barrels or concrete tanks and must be dispensed from wooden or concrete reagent feeders. This precaution is not necessary if the tanks and feeders are cleaned of all precipitate each day, which, however, would obviously be inconvenient.
Zinc Ammonium Acetate.—This reagent was made in the same manner as the copper ammonium acetate. That is, an excess of zinc oxide was placed in a litre bottle, 100 grams of ammonium acetate and 100 c.c. of aqua ammonia added, and the whole made up to one litre. The resulting solution contains about 0.5 per cent Zn, presumably combined as Zn(NH3)4(C2H3O2)2.
The writer has never used this solution in commercial flotation work, but apparently it can be prepared without difficulty by a method similar to that used in making the copper ammonium acetate solution.
Amyl alcohol was used in some tests for frothing purposes because it seemed more selective than pine oil or cresylic acid.
Pine oil used was Yarmor steam-distilled.
Cresylic acid was furnished by the American Cyanimide Company.
Zinc xanthate was prepared by precipitating the salt from a solution of zinc sulphate with potassium iso-amyl xanthate. This precipitate, after being thoroughly washed, was dissolved in aqua ammonia.
Description of Tests
In connection with the descriptions of tests that follow, reference should be made to Table II.
Test No. 4.—This was run with standard reagents for the separation of the chalcopyrite and pyrrhotite. These reagents were:
Soda ash…………………………………..3.0 lb.per ton
Sodium cyanide………………………..0.2 lb.per ton
Copper ammonium acetate………..0.2 lb.per ton
Ethyl xanthate………………………….0.2 lb.per ton
Pine oil…………………………………..0.05 lb.per ton
The procedure was to float-off a large bulk froth with the above reagents and clean this froth twice without addition of reagents. The tailing of the bulk froth was treated with:
Copper sulphate………………………………….1.0 lb. per ton
Ethyl xanthate…………………………………….0.4 lb. per ton
Pine oil……………………………………………..0.05 lb. per ton
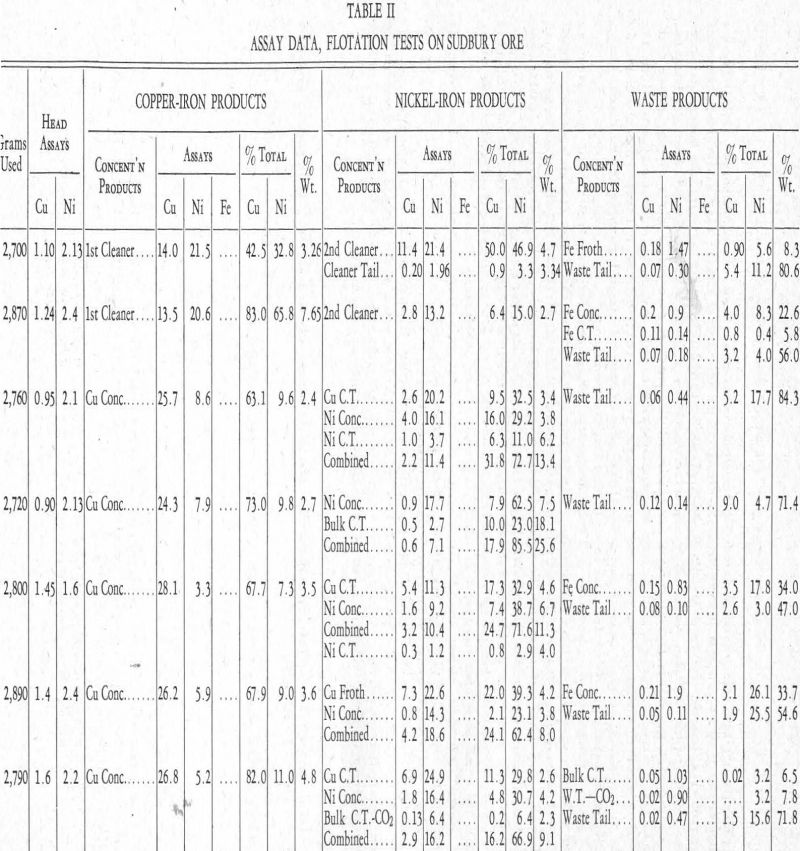
Reviewing this work, the two cleaner-froths show chalcopyrite and pentlandite when the reagents noted are used. The cleaner tailing indicates that the pentlandite is slower in responding than the chalcopyrite. This is also evident in both the iron froth and the waste tailing.
Test No. 5—Finer grinding was the only change in this test as compared with test No. 4. It will be seen from Table II that the only difference in the result was an increased recovery of the nickel, but this was owing to an increase in weight of the mineral put into the first bulk froth, which was from 11.3 per cent to 15.4 per cent, an amount practically equal to the increased nickel recovery. This was the first indication that finer grinding did not release more pentlandite, and it pointed to the possibility of a nickel-bearing pyrrhotite being present, as had been suggested to the writer by C. S. Parsons of the Mines Branch, Department of Mines, Ottawa, before the work here described was started.
Tests Nos. 6 to 16.—Tests Nos. 6 to 16 only confirmed the inability to reduce the nickel in the tailing. These and other tests not included in Table II covered attempts with the use of various compounds analagous to copper ammonium acetate to effect a separation of the chalcopyrite and pentlandite, which for the most part were unsuccessful. Although the solubility of nickel xanthate in cyanide is counteracted by addition of copper ammonium acetate, the effect is not so great as in the case of the copper xanthate which, under these conditions, is practically insoluble. As has been stated, also, zinc ammonium acetate delays or prevents the formation of nickel xanthate, presumably by forming a zinc xanthate and keeping nickel xanthate in solution in ammonia. This indicated the possibility of floating the chalcopyrite but not the pentlandite.
Test No. 17.—This test was run with reagents in whose presence, as shown by the chemical investigation, nickel xanthate is not formed. The reagents were:
Soda…………………………………………..2.0 lb. per ton
Sodium cyanide…………………………..0.2 lb. per ton
Zinc ammonium acetate……………….0.5 lb. per ton
Zinc xanthate……………………………….0.4 lb. per ton
This floated-off what was supposed to be a copper froth which, upon cleaning, resulted in a copper concentrate and copper cleaner tailing.
The second froth was taken off with ethyl xanthate and cleaned, making a nickel concentrate and nickel cleaner tailing.
The whole operation seemed to lack the extra acceleration that is given both chalcopyrite and pentlandite by copper ammonium acetate. This is shown by the large amount of copper going down into the nickel products and the nickel in the tailing.
Test No. 18.—Appreciating that test No. 17 showed a lack of accelerating force either because of the weakness of the ammoniacal solution of zinc xanthate or because of the lack of copper ammonium acetate, it was decided to change the conditions in an attempt to get all the nickel and copper into the froth and then make a separation of the minerals in the froth. The reagents used were:
Soda ash………………………………….3.0 lb. per ton
Cyanide…………………………………..0.2 lb. per ton
Copper ammonium acetate……….0.2 lb. per ton
Ethyl xanthate…………………………0.2 lb. per ton
Pine oil……………………………………0.1 lb. per ton
The procedure was to pull a bulk froth and clean it immediately in the same reagents. This resulted in a waste tailing and a cleaner tailing.
The second, or bulk cleaner froth, was again cleaned with no additional reagents but zinc ammonium acetate. This resulted in a froth carrying most of the copper and a cleaner tailing carrying the nickel.
Here, as in all the earlier tests, the nickel does not all float out of the tailing and bulk cleaner tailing.
Tests Nos. 21 to 23.—Tests up to this point had indicated that a proper concentrate was possible, at least in practice, since the tendency for the chalcopyrite to float away from the pentlandite had been shown in test No. 18. It was now decided to place all the emphasis on the recovery of the nickel from the waste and bulk cleaner tailing.
Tests Nos. 21 and 22 differed from No. 18 only as regards the xanthate used. In No. 21, an ammoniacal solution of zinc xanthate was used, and in No. 22 an ammoniacal solution of nickel xanthate. Both of these tests showed reasonable grades and recoveries of copper concentrates. The one difficulty was the tailing nickel values.
Test No. 23, which is not included in table II, was run by making the solution acid with acetic acid in an attempt to get conditions in the flotation cell right, chemically, for the formation of nickel xanthate. However, pyrrhotite floated as well as pentlandite.
Tests Nos. 24 to 32.—A series of tests was now made in an endeavour to find a condition wherein the chemical relationships would allow the formation of nickel xanthate, and therefore the flotation of pentlandite, without allowing the formation of iron xanthate, which would cause the pyrrhotite also to float. An attempt was made to find an acid that could be used with cyanide, in conjuction with the alkaline reagents indicated chemically, and that would allow the formation of nickel xanthate, and thus the flotation of the pentlandite, without destroying the effect of the cyanide on the pyrrhotite. In other words, allow nickel xanthate to precipitate but iron xanthate to remain in solution; but although various acids were tried, no successful combination was found. In test No. 32, for example, CO2 was used in place of air in the agitation of the pulp, the idea being that the CO2 would give an acid reaction and so be more favourable for the precipitation of nickel xanthate. The reagents used were:
Soda ash……………………………….3.0 lb. per ton
Cyanide………………………………..0.2 lb. per ton
Amyl xanthate……………………….0.2 lb. per ton
Copper ammonium acetate……..0.2 lb. per ton
Pine oil………………………………..0.05 lb. per ton
A bulk froth was taken off followed by the introduction of CO2 gas in place of air into the solution until it was acid. A froth came off which contained 3.2 per cent of the total nickel. This, however, was of no significance as the froth assayed only 0.9 per cent Ni and was all pyrrhotite, evidently nickel bearing.
At the end of the usual bulk cleaning, CO2 was run in place of air and the resulting froth assayed 6.4 per cent Ni and contained 6.4 per cent of the nickel. In this instance the effect of the CO2 was evident. However, results in an alkaline circuit, where the control was not so delicate, were preferred.
Tests Nos. 33 to 40.—At this point, a number of tests (Nos. 33 to 40, not included in Table II) were run, using a variety of reagents, as aerofloat, ammonium butyl-di-thio-phosphate, flotagen, and a number of others. While they all proved worthy of consideration, none were found with any advantage over xanthates, and especially fresh xanthates.
Test work had shown that zinc ammonium acetate will retard the flotation of pentlandite in the presence of chalcopyrite without the use of cyanide or copper ammonium acetate (Figure 8). This is no doubt because nickel xanthate is not formed, or is formed at a slower rate than copper xanthate, in the presence of zinc ammonium acetate; also because nickel xanthate is more soluble than copper xanthate in ammonia.
Test No. 43.—Test No. 43 was run to try out the indicated use of various reagent combinations using sodium pyrophosphate. The reagents were:
Sodium pyrophosphate………………………………0.7 lb. per ton
Amyl xanthate…………………………………………..0.4 lb. per ton
Amyl alcohol……………………………………………..0.1 lb. per ton
These reagents were sufficient to float a bulk froth containing chalcopyrite and pentlandite away from the pyrrhotite and gangue. After the frothing stopped, 0.2 lb. per ton of copper ammonium acetate was put in and a scavenger froth, called special froth, was pulled off. This was to determine whether or not the addition of copper ammonium acetate was necessary. The small amount of both nickel and copper recovered in this special froth indicates that it was not.
The froth pulled off by the above reagents was next cleaned with the following:
Ammonia………………………………………….3.0 lb. per ton
Cyanide…………………………………………….0.2 lb. per ton
Copper ammonium acetate…………………0.2 lb. per ton
Amyl xanthate……………………………………0.1 lb. per ton
Amyl alcohol………………………………………0.1 lb. per ton
The relatively large amount of ammonia used makes it doubtful whether this method would be practicable in commercial work. It is of interest to note, however, that the flotation result confirmed the chemically indicated results (Figure 8, column III). The cyanide and copper ammonium acetate ensured the flotation of the chalcopyrite. If not affected by another reagent, the pentlandite also would be floated off, but the xanthate of nickel is soluble in ammonia so its formation is prevented.
Test No. 44.—Test No. 44 is a duplicate of No. 43, but using less ammonia. The copper concentrate is poorer. The grade of the nickel concentrate is higher, but the recovery is poorer. Otherwise, the results are what would be expected.
Test No. 45—The first part of test No. 45 was run with the same reagents as in tests Nos. 43 and 44. Inasmuch as sodium pyrophosphate allows the formation of nickel xanthate in as strong an alkaline solution as cyanide, it was thought that if the excess sodium pyrophosphate were removed it would be easier to retard the flotation of the pentlandite. With this in mind, lime was put in to form, if possible, calcium pyrophosphate, which would be an inactive salt as far as the nickel xanthate was concerned. A distinct trend towards a good separation was noticed: although the copper concentrate runs much too high in nickel, the recovery of nickel in this concentrate is one-third that of the copper. This froth could be easily cleaned to make a marketable concentrate, indicating a possible direction for further work.
Tests Nos. 46 to 60.—A series of tests (Nos. 46 to 60) was now run in an endeavour to cut down the excessive amount of ammonia. However, none of these showed anything different from the trend of test No. 45. Some work was done in an attempt to use a lead salt, as lead acetate, to react with the xanthate in excess of what would be necessary to react with the copper salt from the chalcopyrite. Lead xanthate is between copper xanthate and nickel xanthate in solubility (see Figure 8), and it was thought, for this reason, it would react with all the xanthate not reacting with the copper, and in this way prevent the formation of nickel xanthate. The results were quite comparable with those of the other schemes as represented by tests Nos. 43, 44, and 45.
Test No. 61.—In test No. 61 an attempt was made to float the pentlandite ahead of the chalcopyrite. It was based on the fact that nickel xanthate is not soluble in cyanide in the presence of sodium pyrophosphate, whereas copper and iron xanthates are soluble, or are not formed. The procedure was to make a bulk float, clean the bulk froth, and then make the separation of the pentlandite and chalcopyrite. This method was adopted because it was necessary to get as much pyrrhotite as possible away from the chalcopyrite and pentlandite before the cyanide was used, in order to avoid an excessive consumption of the latter when the chalcopyrite-pentlandite separation was tried. By floating, as outlined above, a waste tailing is made which contains pyrrhotite and gangue, and a cleaner tailing which is mostly pyrrhotite. These two waste products contain the greater part of the pyrrhotite. In this connection, it will be seen that in separating two minerals in a bulk froth, no matter which mineral is floated off first, the second mineral, if left with the tailing, will be low grade because it will be concentrated only by taking the first mineral away from it. This fact favours the flotation of the pentlandite first, because the chalcopyrite-pyrrhotite separation, which would be necessary in order to have high-grade copper concentrate, is simpler than the pentlandite-pyrrhotite separation, at least with the reagents used in these tests. Reagents for the first bulk float were:
Soda ash…………………………………..2.0 lb. per ton
Sodium pyrophosphate……………..0.6 lb. per ton
Amyl xanthate…………………………..0.1 lb. per ton
Amyl alcohol……………………………..0.1 lb. per ton
This reagent combination served to make a bulk float of the chalcopyrite-pentlandite, which froth was again cleaned in a similar reagent condition or environment, giving, up to this point, two tailing products—a waste tail and a bulk cleaner tail—as well as a froth.
The froth from the second cleaning was treated with
Sodium pyrophosphate…………………….0. 7 lb. per ton
Sodium cyanide………………………………..0.2 lb. per ton
Amyl xanthate…………………………………..0.1 lb. per ton
Amyl alcohol…………………………………..0.08 lb. per ton
This resulted in the flotation of a froth containing most of the pentlandite. This froth should have been cleaned, as it was in the tests that followed. The tailing from this chalcopyrite-pentlandite separation test was treated with the following reagents to effect a separation of the remaining chalcopyrite and the pyrrhotite that was left from the bulk cleaning operation:
Sodium cyanide………………………………………0.1 lb. per ton
Copper ammonium acetate……………………..0.2 lb. per ton
Amyl xanthate………………………………………..0.1 lb. per ton
Amyl alcohol………………………………………..0.05 lb. per ton
Referring again to the metal xanthate solubilities (Figure 8 and Table I), and considering the reagents used in the three stages of this flotation operation, we note that the first set of reagents would keep iron xanthate in solution in sodium pyrophosphate. The second set keeps the iron xanthate in solution or precipitates a carbonate-cyanide hydrate on its surface, and, in addition, the use of cyanide puts the copper xanthate into solution while the nickel xanthate remains precipitated. In the third step, copper ammonium acetate is added, which makes the copper xanthate as well as the nickel xanthate insoluble, but it is still impossible to precipitate iron xanthate, thus allowing the chalcopyrite to float away from the pyrrhotite.
This test and the five that followed it seem to afford additional evidence of the writer’s contention that metal xanthate solubilities control the flotation of the minerals. Other differential flotation work, in the writer’s opinion, confirms this view; but when a new flotation scheme is predicted by simply investigating the solubilities of the xanthates of the metals to be considered, and then is found to work, the case is much more convincing. This is doubly true in the present instance, because the natural tendency, judging from the work of others on this type of ore, is to float the chalcopyrite first. However, the chemical work had shown that nickel xanthate will precipitate in the presence of sodium pyrophosphate from a cyanide solution, while the xanthates of iron and copper will remain in solution or not form, and this chemical theory was then applied successfully in the flotation machine which contained copper, nickel, and iron minerals. This made possible the flotation, first, of the pentlandite (nickel mineral) and, then, of the chalcopyrite (copper mineral). That this sequence is not natural is indicated by the fact that nickel xanthate is more soluble than copper xanthate, as shown in Figure 8, Column I. This again indicates that metal xanthate solubilities control flotation.
Test No. 63.—The same reagents and procedure as in test No. 61 were used in test No. 63, except that pine oil was the frothing agent instead of amyl alcohol. The first nickel froth, also, was cleaned, bringing out again the accelerated rate of flotation of the pentlandite over the chalcopyrite when these reagents are used.
Tests Nos. 64 and 65.—Because of the small bulk of the concentrates, it was decided to run three rougher tests, combine the rougher froths, and make the separation on these combined froths. In these tests (Nos. 64 and 65) the reagents and procedure were the same as in Nos. 61 and 62, both pine oil and amyl alcohol being used as frothers, and the results obtained were the same.
In the first series of tests, i.e., up to and including No. 32, attempts were made to lower the losses of nickel in the tailing. Finer grinding did not effect this, and it was decided that chemical conditions within the cell were not favourable to the precipitation of a nickel xanthate. However, the absence of cyanide and presence of sodium pyrophosphate, conditions existing in tests Nos. 64 and 65, allow complete precipitation of nickel xanthate.
The amount of nickel present in the waste tailing and the bulk cleaner tailing, which comprise 80 per cent to 85 per cent of the weight of the ore, was reduced from between 25 per cent and 15 per cent by using sodium pyrophosphate in place of cyanide to keep the pyrrhotite down. This indicated that further recovery was not to be looked for from the chemical standpoint.
In order to determine if finer grinding would be an aid, test No. 65 was allowed to stay in the ball-mill twice as long as No. 64. Both grinds were all minus-200 mesh, but as no elutriator was available, no attempt was made to get an exact size comparison, it being considered that doubling the time in the ball-mill would ensure a sufficient difference in grind to indicate if lack of fineness of grind was the cause of non-success in the attempt to lower the tailing assay.
Results as far as lowering the tailing was concerned, were nil, which is a definite indication that the pyrrhotite mineral itself contained about 0.7 per cent Ni, which, of course, would not be released by fine grinding.
The poor concentrate grades of test No. 65 are attributed to oxidation of all products during the excessive time necessary to complete the test, which was about ten hours. This effect of oxidation could possibly be counteracted in practice.
Test No. 69.—In test No. 69, first froth reagents were put in the ball-mill to see what resulted; but nothing of importance materialized. Reagents and procedure were substantially the same as in tests Nos. 64 and 65.
Test No. 72.—In the last test (No. 72) recorded in Table II, using substantially the same procedure and reagents as in tests Nos. 64 and 65, an attempt was made to make a higher-grade copper concentrate by two cleanings. The grade was fair, but the number of products formed reduced the recovery to a point that made the test look poor. It is work of this kind that shows the necessity of larger-scale or pilot-mill test work, in which the bulk of these middling products should be considerably reduced and better grade concentrates result.
Calculated Estimate Indicated by Test Work
While the tests that have been described in some detail in the foregoing pages do not show any startling results, they do indicate a tendency towards results that could be obtained either by larger-scale work or on ore containing more of the minerals in question.
The tests bring out two important points. One is that a low tailing can be made consistently, and another is that high-grade concentrates are possible. Larger-scale test work would allow cleaning operations on sufficient material to put more of the mineral in the concentrate and less in the middling products.
At the foot of Table II, the writer has included what he considers a fair estimate of what could be done, on fresh ore, on larger-scale test work or pilot-mill work.
The head assays in this estimate are somewhat higher than the average, the copper especially, and on lower-grade material lower recoveries would result, because tailing assays are reduced to what can be considered almost an irreducible minimum. This is more true of the copper than of the nickel, because extremely fine grinding did not result in a reduction of the nickel— an indication that some of the nickel is present in the ore as a pyrrhotite containing 0.7 per cent nickel, as the work of others, notably C. S. Parsons, has suggested.
Resume of Results of Tests
- The test work on the Sudbury Ni-Cu-Fe ore indicates that part of the nickel present in the ore experimented with is probably combined in the pyrrhotite, or in solid solution with it, to the extent that a pyrrhotite concentrate will assay about 0.7 per cent Ni.
- It is possible to produce a nickel concentrate that will assay well over 20 per cent Ni.
- The recovery of nickel is good if applied to the nickel mineral other than nickel-bearing pyrrhotite, assumed to be present.
- Copper concentrate of both good grade and good recovery is possible.
- Reagents worked out to cope with this problem make it possible to float the copper minerals away from the nickel minerals, or, alternatively, to float the nickel minerals away from the copper minerals.
- No cost and recovery data were available which would have made it possible to determine the economic advisability of using the concentration schemes as outlined here in preference to present practice of smelting.
- Results as outlined here are offered only as directions to be followed in continuous test-plant experiments or pilot mill work.
Gold-Pyrite Separation
The usual procedure for the concentration of gold in a pyrite-chalcopyrite-gangue ore is to treat the pulp with lime and cyanide and float off a chalcopyrite froth containing as much of the gold as possible. This has never been entirely successful. In the light of the chemical data shown in Figure 8, it seems that, under certain conditions, flotation of the gold is not aided by xanthate in the presence of cyanide, and one of these conditions seems to be the flotation procedure outlined above for Ni-Fe-Cu ores. That is to say, gold xanthate is soluble in an alkaline solution of cyanide and, therefore, if the writer’s theory is correct, it will not float under such conditions except as a mechanical entanglement or attachment with copper minerals.
The chemical data in Figure 8 also show that gold xanthate is insoluble in the presence of sodium pyrophosphate, while iron xanthate is soluble.
An attempt to apply the chemical data to a gold ore from the Rouyn district resulted in a quite clean separation of the chalcopyrite-pyrite-gold in a froth and the pyrrhotite-gangue in the tailing. At the time, other work prevented the writer working out a flow-sheet in detail, but he was convinced that a separation of the chalcopyrite and gold from both the pyrite and the pyrrhotite could be made, provided, of course, the copper is present as chalcopyrite and not as cupriferous pyrite.
At a later date this gold-pyrite separation was studied by L. W. Buchanan, under the direction of the writer, at the Colorado School of Mines. A brief account of his work follows:
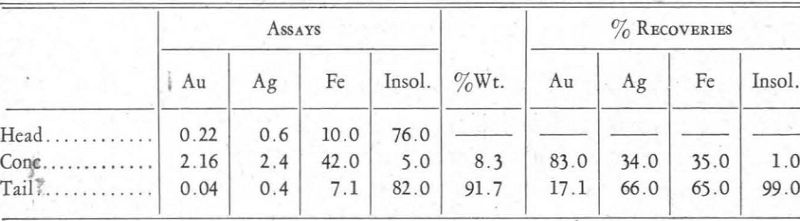
The ore treated assayed as follows:
Au…………………………………………………0.22 oz. per ton
Ag…………………………………………………0.6 oz. per ton
Pb…………………………………………………0.3 per cent
Cu…………………………………………………0.5 per cent
Zn…………………………………………………0.7 per cent
Fe…………………………………………………10.0 per cent
Insol…………………………………………………76.0 per cent
Sodium pyrophosphate gave poorer results than other combinations, so tests with it will be disregarded. As in the case of the Rouyn ore and the Sudbury ore, the tests seemed to indicate that the fast-oxidizing pyrrhotite will respond to sodium pyrophosphate more quickly than will pyrite.
After a scheme of manipulation and reagents had been worked out, the tests gave results about as shown in the table on page 221.
As has been stated, it is the writer’s contention that, if it is desired to float a mineral or metal, conditions must be right for the formation of a precipitate of a xanthate of the metal in question; and, further, that this mineral or metal must be sufficiently soluble to allow of a chemical reaction between its soluble products and the flotation reagents.
It is entirely doubtful that gold, even in the presence of ferric and cupric salts, is sufficiently soluble to meet the requirements stated.
In order that these requirements might be met, the ore was conditioned for ten minutes with soda ash and cyanide, the soda ash acting as a deflocculator and a protective alkali, while the cyanide freshens up the surfaces of the gold and encloses it with a film of soluble gold cyanide which is available for further reactions.
After conditioning, zinc ammonium acetate and zinc xanthate in solution in ammonia were added. The former, being only slightly dissociated, counteracts the free cyanide, with which it reacts much more readily than with the gold cyanide; the latter provides a xanthate radical which is available for metals above zinc in the electromotive series (e.g., gold and copper) and not for metals lower than zinc (e.g., iron). Result: flotation of the gold, chalcopyrite, and lead.
Resume
(a) Au first made soluble in NaCN.
(b) NaAu(CN)2 changed to gold xanthate and zinc cyanide, because the excess cyanide reacted with the zinc ammonium acetate, i.e., production of a xanthate insoluble in zinc xanthate and zinc ammonium acetate.
(c) Iron xanthate either soluble or not formed because of a previous coat of carbonate-cyanide-hydrate.
The results obtained in this test showed a distinct concentration of the gold in preference to the pyrite—a recovery of better than 80 per cent of the gold and only 35 per cent of the iron.
This work is interesting from the practical standpoint as well as for the reason that the flow-sheet was outlined from a consideration of metal xanthate solubilities before the test work was started.
It may be of interest to note here that a possible method of separating nickel and cobalt minerals is suggested by a statement in Prescott and Johnson’s Qualitative Analysis, which says: “A solution of potassium xanthate precipitates neutral solutions of nickel and cobalt, the former being soluble in ammonium hydroxide”. The writer has never tried this, but it certainly is a lead to follow. It would be necessary, of course, for the nickel and cobalt to be present in the ore in separate minerals.
Conclusions
- Investigation has shown that the xanthates of the heavy metals differ greatly from one another in their solubility in the various flotation reagents that are in common use and in others that might be used.
- Common practice in ordinary differential flotation of galena-chalcopyrite-sphalerite-pyrite can be explained by chemical data on the solubilities of metal xanthates.
- A method for the separation of pentlandite-chalcopyrite-pyrrhotite flotation which had been outlined from purely chemical data was confirmed by subsequent flotation test work.
- Failure to float gold with chalcopyrite in the ordinary practical separation is explained by the solubility of gold xanthate in cyanide.
- A method for the separation of pyrite from gold-chalcopyrite was outlined from chemical data and confirmed by subsequent flotation test work.
- Chemical data indicated that pentlandite could be floated ahead of chalcopyrite or, alternatively, that chalcopyrite could be floated ahead of pentlandite. This was confirmed by flotation test work.
- The minerals of ores that have been exposed to the influence of oxidation or secondary precipitation do not respond to the treatment outlined as well as do clean unaltered minerals.

