Among the wide variety of modern surface chemistry analytical techniques, contact angle measurements still remain attractive for wetting characterization of solid surfaces. It is demonstrated that the contact angle value depends on the technique used when nonideal, heterogeneous and rough, solid surfaces are examined.
Reagents and Materials
Three different solid surfaces were used in the experiments: 1) a polyethylene (PE) film on a glass slide or an aluminium plate prepared by coating the slide/plate with low-density polyethylene (Scientific Polymer Products, Inc.) in toluene solution and evaporation of the solvent, 2) optical-grade quartz plates (Minarad Scientific Inc.) hydrophobized with a solution of chlorotrimethylsilane in cyclohexane, 3) a gold film, which was vapor deposited onto a silicon wafer, and covered with a thiol self-assembled monolayer of dodecanethiol.
The chemicals used in the experiments were as follows: distilled and Milli-Q deionized water with pH=5.8+0.1, specific conductivity <10 -6 S/cm, and surface tension 72.6+0.2 mN/m at 20°C; ethylene glycol with purity more than 99.5% (Mallinckrodt, Inc.) and surface tension 47.8+0.2 mN/m (20°C); spectrograde cyclohexane, toluene, and acetone (Mallinckrodt, Inc.); chlorotrimethylsilane with purity more than 98% (Mallinckrodt, Inc.); 1-dodecanethiol with 98% purity (Aldrich Chemical Co.); dehydrated ethyl alcohol (Quantum Chemical Co.); and chromic-sulfuric acid cleaning solution (Manostat).
Solid Surface Preparation
Granulated low-density polyethylene (PE) was dissolved in toluene at a temperature of 60-70°C (0.2-1 wt% concentration). The microscopic glass slide or aluminium plate was covered by the PE-toluene solution and kept in a clean vacuum oven several hours at a temperature of 60-65°C. The toluene was evaporated and the remaining polyethylene formed a film at the surface of the slide/plate.
Quartz plates were methylated as in previous studies. Carefully prepared, dry quartz plates were immersed into a solution of chlorotrimethylsilane (TMCS) in cyclohexane. The concentration of TMCS in cyclohexane was 1·10 -6-5·10 -6 M and the time of immersion was 10-30 minutes. Hydrophobized plates were washed with cyclohexane to remove unbonded residual TMCS, and were then heated in the oven for 2 hours at 110-115°C.
A 200 nm gold film supported on a silicon wafer, precoated with titanium to improve adhesion of the gold film, was used as a substrate to prepare a model homogeneous surface composed of a self-assembled monolayer of 1- dodecanethiol. The gold surface was cleaned with chromic-sulfuric acid cleaning solution, distilled water, and ethanol. Final cleaning from organic contaminants and/or previously adsorbed thiol monolayers was conducted in a Tegal Co. plasma chemistry reactor (Plasmod model). The substrate was treated with argon plasma for 60 minutes. Such a freshly cleaned gold surface was immediately immersed for about 30 minutes into the 1 mM ethanol solution of 1-dodecanethiol (thiol forms a self-assembled monolayer on the gold surface through gold-sulfur bond with the hydrocarbon chain oriented into the environment). After removal from the adsorbate solution, slides were washed with ethanol and then dried in a stream of argon.
Contact Angle Measurements
Three different contact angle measurement techniques were used in these studies and they are as follows: 1) static sessile-drop technique (Fig.2a), 2) static captive-bubble technique (Fig.2b), and 3) dynamic captive-bubble (drop) technique (Fig.2c).
The static sessile-drop (Fig. 2a) and captive-bubble (Fig.2b) techniques for contact angle measurements were used with a NRL goniometer (Rame-Hart, Inc.). In the sessile-drop technique, the solid sample examined was placed in a controlled-atmosphere Rame-Hart chamber which was partially filled with liquid used in contact angle experiments. The chamber with solid sample remained closed 0.5-1 hours to achieve equilibration between the phases. A liquid drop was introduced onto the solid surface through a microsyringe and the needle remained in contact with the drop. A special precaution was taken in the measurements to avoid distortion of the drop shape by the needle. The three-phase contact line of the liquid drop was made to advance or retreat by adding or withdrawing a small volume of liquid (Fig. 2a) and the advancing and receding contact angles, respectively, were measured after 30-45 seconds on both sides of the drop (the average values are reported). These contact angles were measured for varying drop size.
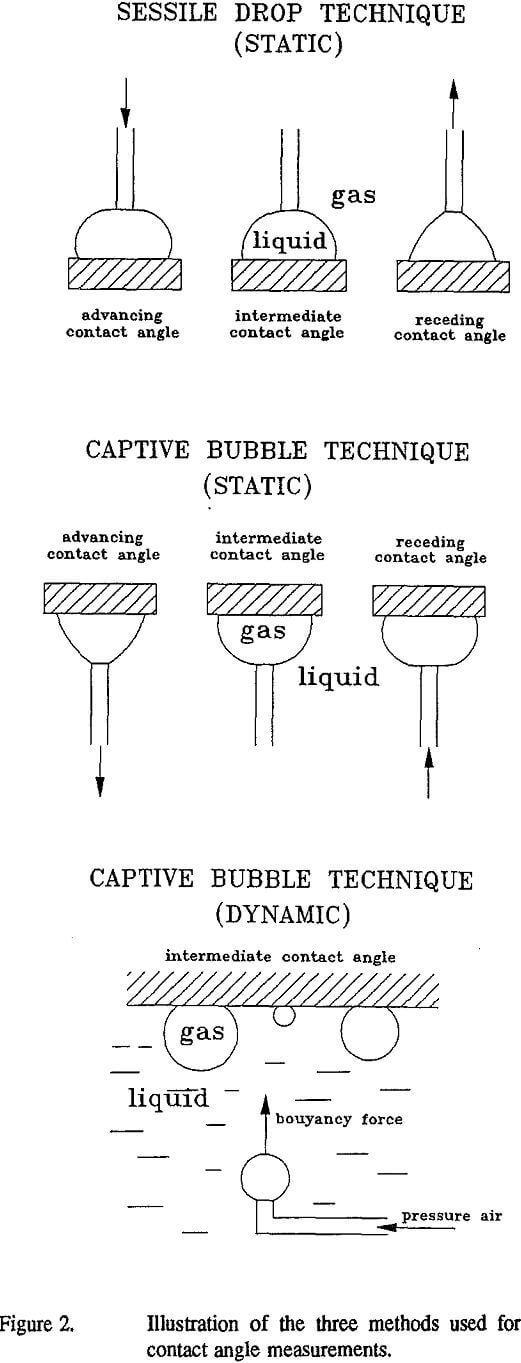
In the captive-bubble technique, the solid sample was placed in the rectangular glass chamber on two stable supports with flat surfaces. The glass chamber with sample was filled with liquid. A small air bubble was made at the tip of a U- shaped needle using a micro-syringe. The gas bubble was attached to the solid surface. The three-phase contact line of the gas bubble was made to advance or retreat by adding or withdrawing a small volume of air (Fig. 2b) and the advancing and receding contact angles (as measured for the liquid phase!), respectively, were measured with the NRL goniometer after 30-45 seconds on both sides of the gas bubble (the average value is reported). The contact angles were measured for varying bubble size. The needle remained in a contact with the air bubble during all measurements but no distortion of the bubble shape by the needle was allowed to affect the contact angle measurements.
As the third technique, the dynamic captive-bubble method (Fig. 2c) has been used for the examination of the effect of bubble size on contact angle. The dynamic captive-bubble technique differs from the static method by the way in which gas bubble attachment occurs at the solid surface. Air bubbles of varying size are generated in the liquid with a syringe under the solid surface and allowed to release from the needle. Released bubbles are captured at the solid surface as a result of bouyant transport and attachment After the air bubbles attach to the solid surface, both contact angle and bubble base diameter are measured with the NRL goniometer. In contrast to the static techniques the contact angle is not always uniquely defined by the dynamic technique, as will be evident from the results to be discussed.
Wetting characteristics of uniform thiol monolayers at smooth gold surfaces, methylated quartz surfaces, and roughened polyethylene surfaces were analysed with the sessile-drop and captive-bubble contact angle techniques. An agreement for advancing contact angles as well as for receding contact angles as determined with two different techniques, sessile-drop and captive-bubble, was obtained for a close-to-ideal system, i.e., a freshly prepared self-assembled monolayer of dodecanethiol on a smooth and pure gold film. Increasing roughness and heterogeneity of the solid surfaces caused differences between the measured receding contact angles when determined with different methods, sessile-drop or captive-bubble. Similar discrepancies between the two experimental techniques, but to a lesser extent, were observed for advancing contact angles.
The contact angle as measured for the air bubble/liquid/solid system, where the air bubble was deposited onto the solid surface with the dynamic captive-bubble technique (injection of gas bubble into the bulk liquid and transport to the solid surface due to the buoyant force) corresponded to the receding contact angle as measured with the static captive-bubble technique for most of the systems examined. On the other hand, when rough and heterogeneous solid surfaces were used, intermediate contact angles (contact angles between advancing and receding) were also observed. These experimental data emphasize a disadvantage of the dynamic technique for which it can be concluded that the contact angle cannot be uniquely defined when non-ideal systems are examined with this technique. However, it must be remembered that a unique advantage of this technique is the ability to examine the contact angle/bubble (drop) size relationship over a wide range of bubble (drop) volumes, from several micrometers to several millimeters in diameter.
With respect to flotation, the receding contact angle measurement is a more relevant indication of floatability. However, the results from these studies clearly show that receding contact angles may differ depending on the method of contact angle measurement. The captive-bubble contact angle method, in our opinion, is rather more appropriate for the flotation systems. Additional research effort should be undertaken to select between the static and dynamic captive-bubble technique. Because of dynamic conditions associated with the attachment of gas bubble at the surface of a mineral particle, the dynamic captive-bubble may provide for better characterization of real flotation systems. However, the dynamic captive-bubble technique which was presented in this contribution is limited to solid plates. Further effort is needed in order to incorporate solid particles into the dynamic captive-bubble methodology.
Only after an appropriate selection of a contact angle measurement technique can any correlation between contact angle and flotation separation performance be established. It is clearly shown in this paper that solid surface imperfections, roughness and heterogeneity, make the contact angle/bubble size relationship nonlinear. The contact angles as measured for large bubbles at mineral surfaces might not correlate with flotation performance results at all. Gas bubbles (sometimes much smaller than those analyzed for the several systems and presented in this contribution) attach to fine solid particles in the flotation process. The contact areas between gas bubbles and solid particles in such flotation systems is even much smaller than the contact areas examined herein. In this regard, the contact angles may differ significantly from those measured for gas bubbles of several millimeters in size.

