Acid mine drainage is one of the most persistent industrial pollution problems in the United States. Over 5,000 miles of our country’s streams and rivers still fail to meet water quality standards due to acid mine water that originates primarily in abandoned mined lands (Kim et al., 1982). Less than 10 percent of this total is associated with drainage from various sulfide mineral operations; most is associated with coal mine operations in the eastern and midwestern states.
Although desirable, it is impractical or impossible at many sites to eliminate the acid drainage at its source in the coal or overburden. As an alternative, Federal agencies, coal companies, and researchers have investigated the feasibility of using wetlands as low maintenance biological systems, to provide long-term low-cost treatment for acid water. The objectives of this paper are: (1) to examine design criteria for constructed wetland systems; and (2) to survey the developing technology of wetland systems for acid mine drainage treatment in the Appalachian bituminous coal region.
In the natural setting, bogs, marshes, swamps, fens, and wet meadows are all considered “wetlands”. Numerous classification schemes have been developed to further define wetland types; the schemes rely on hydrologic processes and vegetation types as primary sorting factors. The ongoing National Wetland Inventory conducted by the Fish and Wildlife Service (Cowardin et al., 1979), for example, defines wetlands as either supporting predominantly hydrophytic vegetation, or having a substrate that is undrained hydric soil or saturated organic material. While some wetlands are transient, such as those located in lakes which have filled with sediment (Wetzel, 1975) or those situated along meandering rivers, other wetlands have existed for thousands of years (Wieder, 1982) and appear to be self-perpetuating systems.
The use of natural wetlands for acid mine water treatment is seldom an acceptable option. First, while it is common to find small natural wetlands recently established on acid water seeps near mined areas (Brooks et al., 1985), it is rare that a long-established natural wetland is present on or adjacent to a permitted mine area (Wieder and Lang, 1984). Second, legal barriers exist which must be overcome before using natural wetlands as mine water treatment systems. On the Federal level, the 1977 Surface Mining Control & Reclamation Act prohibits surface mining within 100 feet of a wetland, and the 1977 Clean Water Act limits discharge of pollutants from mining operations into wetlands. Also, there are cases where wetland vegetation has been killed as a result of mine water inflow of both treated water (Wieder et al., 1984) and untreated water (G. E. Lang, personal communication). Unless it can be demonstrated through research and extensive monitoring that natural wetland systems can remain healthy while effectively treating acid mine water, natural wetlands are unlikely to be used for acid water treatment.
A logical alternative is to construct wetlands where needed as an alternative to chemical treatment facilities. The feasibility of this concept was first demonstrated in pilot-scale tests, and is now being evaluated in full-scale field tests at over 25 mine sites in Pennsylvania, West Virginia, Ohio, Maryland, and Alabama. The Bureau of Mines, along with other Federal and State agencies, is facilitating monitoring and evaluation of the sites. It is expected that an additional 100 wetlands will be built in the Appalachian bituminous coal region during 1986.
Wetlands as Water Treatment Systems
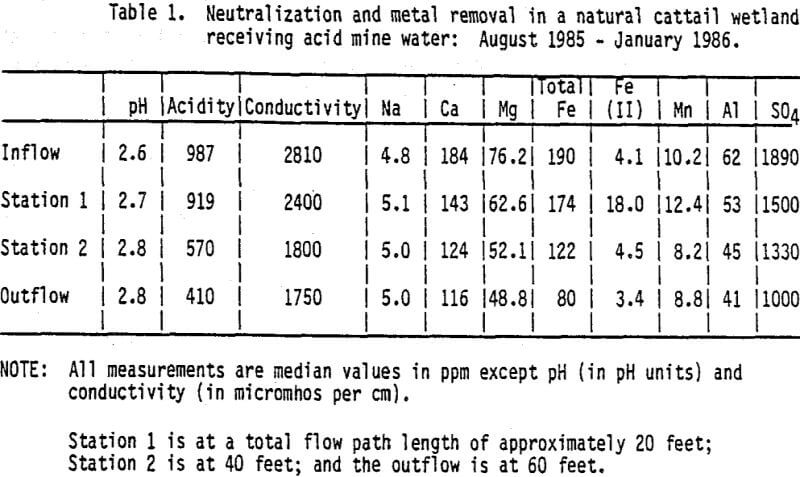
The use of wetlands for water treatment is a demonstrated technology (Chan et al., 1982; Kadlec and Tilton, 1979; Lynard et al., 1980; and Seidel, 1976). Constructed wetlands for acid mine drainage treatment are special applications of wastewater and stormwater treatment technology. Water associated with pyrite oxidation processes contains high acidity, sulfate, metals and suspended solids concentrations. Acidity and sulfate concentrations are often orders of magnitude higher in acid mine water (20-1000 mg/L and 300-3500 mg/L, respectively; see Table 1) than in other water treated in wetland systems (net alkalinity and 10-100 mg/L, respectively; Chan et al., 1982). In wetland systems, acidity is lowered by the removal of metal and hydrogen ions from the water and addition of base cations. “Removal” of hydrogen ions is accomplished through promotion of hydroxyl ion-producing processes such as nitrate uptake by plants, and through support of buffering systems at higher pH levels (such as organic acid buffering at pH 4 instead of metal buffering systems at lower pH ranges; Hemond and Eshleman, 1984). Sulfate is taken up to a limited extent by plants; in the anaerobic wetland environment, sulfate reduction is the most active process removing sulfate, with pyrite, hydrogen sulfide gas, and organic sulfur compounds as end products (Gorham et al., 1984; Wieder and Lang, 1986). Metals are removed from the water through ion uptake and adsorption by the living vegetation, cation exchange with the peat, microbially mediated oxidation/reduction reactions, chelation, and precipitation. Suspended solids settle in the slow-moving water, often removing ions from the system at the same time through adsorption.
Constructed Wetlands: Primary Design Criteria
As in the design of stormwater and wastewater treatment wetlands (Hammer and Kadlec, 1983), primary design criteria for acid water-treating wetlands are based on estimates of system loading rates. They directly determine the maximum treatment efficiency and capacity of the wetlands designed. Influent volume and chemical concentrations in conjunction with treatment rate and required effluent concentrations provide the basis for determining the optimal water volume to substrate volume ratio, flow path length, and detention time within the wetland. Adequate measurement and prediction of influent volume is the most critical of these parameters, as inability to control water flow rates has been a common factor among failures of acid water wetland treatment systems to date. Extreme low flow rates and corresponding low water levels have been found to kill vegetation and to expose anaerobic sediment zones, reoxidizing deposited material and eliminating bacterial populations. At the other extreme, high flows have resulted in scouring of vegetation and microbes, and channelization within the wetland.
Considerable variation in flow rates, however, is expected in runoff from mined sites. A year of baseline data on flow rates from water sources to be treated is necessary for an accurate assessment of the median, range, and frequency of flow rates. Lacking baseline data, an estimate of flow extremes can be made from measurements during high flow periods and low flow periods, reports of periodic flow rate measurements, or estimates of seepage/surface flow expected based on precipitation and infiltration characteristics and flow directions. In many reclaimed areas seeps appear during the years following reclamation; provision is necessary in the wetland design for collection and treatment of these sources as well as active seeps. Also, plans should include collection and redirection of surface flow around the wetlands, or storage for augmentation of flow into wetlands during low flow periods.
The median range and frequency of chemical concentrations in water supplied to the wetland need to be determined, preferably by collection of baseline data representative of seasonal changes. Acidity and metal concentrations are of greatest importance, as death of vegetation has been linked to acidity and metal toxicity. Because high or low chemical concentrations may occur at times of high flow as a result of flushing or dilution, respectively, the relationship between flow and discharge must be defined. High flow rates lead to decreased residence times, shorter treatment times, and less contact area due to channelization.
Hydrogen concentrations are moderated as acid water flows through natural and constructed wetlands (Wieder et al., 1982, and Table 1), but to bring pH within the range of effluent standards (pH 6-9) would require a longer flow path and retention time than that required to bring iron and manganese concentrations to acceptable levels. We suggest, therefore, that pH be increased to meet current regulations using a direct chemical contact method such as that achieved by lining the outflow channel with limestone. It should be noted that possible changes to effluent limits to make them comparable to local unimpacted water chemistry are being discussed in consideration of Clean Water Act authorization (Alfred E. Whitehouse, personal communication).
Optimal water volume to substrate volume ratios, flow path lengths, and detention times for wetland systems constructed to treat acid mine water using different vegetation and substrate combinations are not yet available. However, research that focuses on dominant processes responsible for chemical changes observed is underway in both laboratory and field studies to provide a rational basis for determining optimal wetland size and water detention time.
Laboratory Studies
Studies of maximum uptake and adsorption rates in wetland vegetation have been done under controlled laboratory conditions. Tarleton et al., 1984 found that most of the iron applied to the Sphagnum peat accumulated as an organically bound iron fraction (68%), followed by amorphous and crystalline oxides (30%) and sulfide fractions (1+%); iron was not removed from peat by repeated rinsing with deionized water. A more recent study (Weider and Lang, 1986) examining aluminum and manganese adsorption by Sphagnum peat, in addition to iron adsorption, found that aluminum was also bound in an organic fraction, but that manganese accumulated in an oxide fraction only. Cumulative iron adsorption was related to solution iron concentration by the Langmuir function. Controlled studies applying wastewater containing metals to wetland vegetation have demonstrated large variations in metal uptake and concentration within plant material – between species in the same family, in different phenological stages, and in different parts of the same plant (concentration in seeds, growing tips, or roots, for example; Murphy, 1981). Also, release back into the water may occur from the plant prior to or during senescence and decay.
Bench-scale studies of metal uptake and adsorption rates by vegetation in greenhouse settings which simulate the field setting have provided insight into processes active in metal removal. Gerber et al. (1985) reported iron and manganese removal efficiencies of 65-90% and 10-76%, respectively, by flow through 4.6 meter troughs filled with Sphagnum recurvum. Cation exchange by the moss and metal oxidation by bacteria were concluded to be the dominant removal mechanisms, although their relative activities were not assessed. Duplicating the environment and plant density of larger plants, such as cattails, has posed problems of scale difficult to address in greenhouse experiments.
Field Studies
Two independent studies of sites where acid mine drainage was flowing into naturally-occurring Sphagnum moss bogs represent the earliest field studies of treatment efficiency. Huntsman et al. (1978) found Sphagnum recurvem growing in pH 2.5 water in Ohio’s Powelson Wildlife Area. Iron, magnesium, sulfate calcium and manganese all decreased while pH increased from 2.5 to between 4 and 6 as the water flowed through the bog. A natural outcrop of limestone located at the downstream end provided sufficient neutralization to raise the effluent pH to between 6 and 7.
A similar study (Wieder et al., 1982) was conducted in a Sphagnum bog in northern West Virginia. Within 20-50 meters of the inflow border, pH increased from 3.05-3.55 to 5.45-5.05, while within 10-20 meters sulfate concentrations decreased from 210-275 mg/L to 5-15 mg/L and iron decreased from 26-73 mg/L to less than 2 mg/L. Bog effluent chemistry was similar to that of nearby streams unaffected by mine drainage.
Following publication of these pioneer studies, pilot scale field tests were initiated (e.g. Harris et al., 1984, Brooks et al., 1985) on wetlands constructed by mining companies for acid drainage treatment. Wetland construction included augmentation of small natural wetlands that had appeared on mine sites, to increase flow-through time and to extend the wetted area. At other sites, wetlands were constructed in shallow, excavated areas or remodelled sediment ponds. Treatment efficiencies of these wetlands are currently under study. Preliminary data have been presented at several mining, reclamation, and wetlands conferences since 1983 (Huntsman et al., 1985; Wieder et al., 1985; Stone and Pesavento, 1985). In general, constructed wetland treatment systems that have an influent iron concentration less than 30 mg/L and an influent manganese concentration less than 15 mg/L consistently reduce both iron and manganese to less than 2 mg/L. At many sites, much higher iron concentrations are being treated by the wetlands, but only at a few are the iron levels being reduced sufficiently to meet the discharge criteria of 3 mg/L. The highest average iron concentration being successfully treated to that level, at present, is 85 mg/L, though influent concentrations up to about over 100 mg/L are being reduced to 10-20 mg/L (unpublished data).
Manganese removal in these wetlands is inconsistent. One wetland with an average influent concentration of 36 mg/L lowers manganese to 2 mg/L, but at other sites, influent and effluent manganese concentrations are virtually equal. Manganese removal is spatially and temporally variable at some sites while other sites show almost no perturbations. Sphagnum moss, while very effective in removing iron, is ineffective in removing manganese (Wieder and Lang, 1986).
As is apparent, metal ion removal and neutralization rates in constructed wetlands have not yet been quantified. Determination of surface and subsurface water flow rates through constructed wetlands, which govern residence time and areal exposure requirements, is also in the early stages of research. Although considerable study of the hydrology of natural wetlands and hydraulic conductivity of wetland peat has taken place since the 1940’s (see Ingram, 1967 and Rycroft, 1975a and b), variability has been found to be great without concurrent identification of the factors explaining the variation. Also, there is question as to whether Darcy’s Law applies to water flow in peat (Ingram et al., 1974; Hemond and Goldman, 1985). As a consequence, flow rates derived from hydraulic conductivities are of questionable validity in constructed wetlands. Current research using ion tracers in natural Sphagnum moss wetlands (Girts et al., 1985) may provide flow rate estimates and techniques appropriate for use in constructed Sphagnum wetlands. Until this research and similar studies in wetlands with other vegetation communities are completed, however, optimal water volume to substrate volume ratios, flow path lengths, and detention times needed for design purposes given chemical loading rates are not available.
Constructed Wetlands: Secondary Design Criteria
Secondary design criteria include the sediment load of influent, the site limitations imposed by size and shape of area available for wetland construction, the volume and chemistry of groundwater seepage into proposed wetland areas, the soil chemistry (particularly nutrient and metal ion concentrations and acidity), and identification of wetland vegetation in the area. These design criteria are considered “secondary” in that, while they effect treatment efficiency, manipulation of these factors cannot increase the maximum treatment capacity.
- High sediment loads need to be treated in settling ponds before water flows into the wetlands, particularly in Sphagnum moss systems. Elevated mineral soil proportions in organic peat have been associated with differences in natural wetland vegetation communities (Wieder, 1982) as well as plant mortality and reduced water storage capacity of the wetland basin.
- The wetland shape is determined by the area available. To maximize flow path length and detention time, a series of rectangular ponds or channels are more space-saving than a design simulating the irregular perimeter of a natural wetland. On the other hand, if flow can be spread over the entire wetland width, an irregular perimeter in a large (½ acre or more) wetland can increase flow path length and detention time, provide water storage space during high flow periods, and encourage wild life access to the wetland (Brooks, 1985).
- Host wetlands constructed are located to avoid all groundwater and surface water sources other than the acid mine drainage to be treated to control Inflow chemistry and volume. Where contaminant concentrations are high but flow volume is low, it may be preferable to dilute the acid water with a buffered water.
- Soil chemistry may increase buffering capacity in the water to be treated, or it may add to metal and acid loads through leaching; imported top soil and fertilizer are occassion- ally applied to avoid the latter effect.
- Natural wetlands on the mining sites provide supplies of vegetation for small-scale wetlands, especially if they have been exposed to acid and high metal-containing water over time to promote development of tolerant populations. However, in order to preserve natural wetlands, we encourage nursery operations to supply wetland vegetation. Sphagnum moss is particularly difficult to obtain due to its slow growth rate.
Constructed Wetlands: Current Designs
Seeps with median flow rates totalling 1 to 15 gpm often enter the wetland directly, with no inflow control structure. Higher flow seeps are usually collected into a ditch or pond and fed through a channel with a slide gate, weir, level spreader or through a pipe so that inflow can be controlled. High flow streams may be redirected using a channel with or without a slide gate. To avoid channelization of the wetland, flow dispersion structures such as overflow basins, planting of cattails or wetland shrubs at the inflow point, and placement of substrate “islands” near the inflow point have been used. Pumped inflow is generally not required for wetland systems. The most common form of outflow structure is the channel. A gate or weir at some sites provides water level and detention time regulation. Occasionally, a wide diameter standpipe has been used as primary outflow structure, or to augment the primary outflow device in regulating water levels during times of high uncontrolled inflow.
Vegetation of a constructed wetland is generally selected based on the inflow rates and water levels, the chemical constituents of the influent, and natural wetland vegetation found in the region. Vegetation which can tolerate a foot or more of water such as cattails, horsetails, and some bulrushes, also have been used where frequent high flow rates are expected. It should be noted, however, that water treatment efficiency has been observed to decline at high flow rates (corresponding to low contact and residence times). Mosses and wet meadow vegetation are better adapted to low flow rates and water levels at the substrate or moss surface, especially during wetland establishment.
Research and monitoring programs have found vegetation to vary in tolerance of ambient metal concentrations and pH, and in uptake of metal ions (Chan et al., 1982, Warburton et al., 1985). Currently, approximately two-thirds of the constructed wetlands are planted with cattails, and onethird are planted with Sphagnum moss. Species found in the region are generally used in plantings; however, due to increased protection of natural wetlands and a scarcity of greenhouse suppliers of wetland vegetation in the East, some plants must be imported from Wisconsin, Michigan or Minnesota.
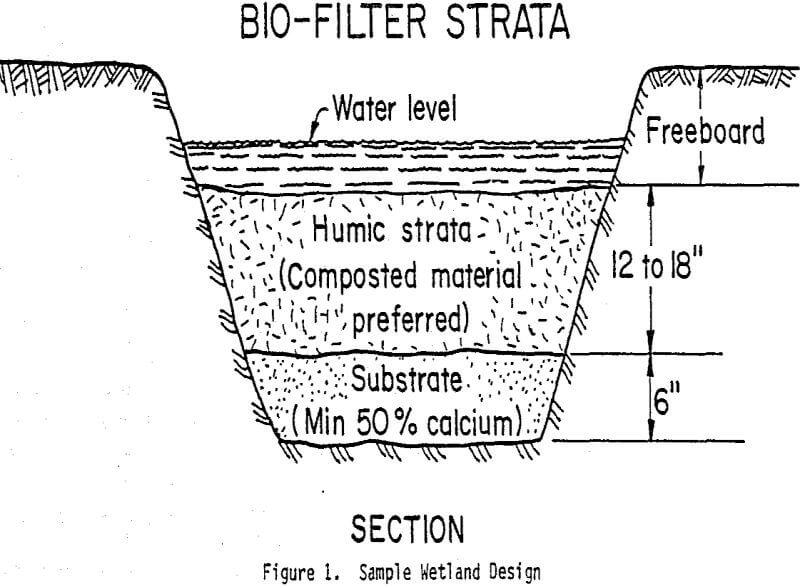
Substrate use is generally dictated by the vegetation to be planted. Cattail and horsetail wetlands have been planted in layered beds of composted hay and manure on top of limestone (Figure 1), or have been planted directly into the mineral soil. Moss wetlands have used hay or peat as underlying organic substrate. Soils are occasionally imported from natural wetlands, with the additional advantage of providing a vegetation seed bank.
Shapes of the constructed wetlands range from a modified sedimentation pond to a series of wide ditches. Control of flow routing and flow rate is also increased with coves, peninsulas and islands. Although these structures are found most frequently in midwest wetlands constructed on mined lands for reclamation purposes other than water treatment (Lawrence et al., 1985), they are currently being incorporated into Appalachian acid water treatment wetland designs. Many wetlands have been designed to provide at least 200 square feet of treatment area per gpm of flow, a rule of thumb assume to be conservative but as yet unsubstantiated.
Along with the critical question of optimal loading rates, evaluation of the long term effectiveness of constructed wetlands in treating acid mine drainage needs to be addressed. With no constructed systems older than five years, the relationship between loading rates and treatment efficiency and capacity over time, encroachment of volunteer (not planted) vegetation, and sedimentation of the systems need long-term study. The importance of long-term monitoring programs of influent and effluent chemical concentrations and volumes, and observation of vegetation and substrate changes during the use of the wetland for treatment is evident to elucidate removal mechanisms. In effect, the monitoring program is the major maintenance expense, as maintenance activities such as drying, scraping of the substrate, periodic fertilizer application, or substrate regeneration have not been necessary. The expenses of wetland treatment of acid mine water are thus limited to initial design and construction costs, and subsequent monitoring.
Summary
The further development of wetland construction technology for treatment of acid mine water awaits definition of optimal loading rates for different vegetation and substrate types, and evaluation of the long-term stability and effectiveness of the system. Research to date indicates that with flow control, metal, sulfate, and acidity removal can be expected in an established constructed wetland. As an inexpensive and low maintenance water treatment technique, we expect that research and development in this field will of necessity increase in the coming years to meet the demands of mining companies and government agencies.