For the commercial production of iron ore pellets from hematite ores, as it is practiced in various pellet plants, the addition of lime has shown to be an efficient means with a view to improving cold compression and abrasive strength of the indurated pellets considerably. Unfortunately, it has also been shown at the same time, that the metallurgical properties as, for instance, swelling behavior, softening during reduction under load and disintegration of the grain, can be adversely affected to a high degree.
Test Methods Employed for Determining the Metallurgical Properties of Pellets
In order to ascertain the metallurgical behavior of iron ore pellets, we carry out, amongst others, certain tests with a view to determining
- the swelling of pellets,
- the behavior during reduction under load,
- the disintegration of the grain in the low-temperature range in a weakly reducing atmosphere.
Swelling test and pressure-reduction-softening test may be con sidered as test methods which reproduce the behavior of pellet in the lower part of the blast furnace shaft. It is possible to carry out isothermal tests at about 1,000 deg. C, since the temperature gradient in modern high-capacity blast furnaces shows a distinct isothermal zone above 900 deg. C.
Effect of Lime Addition upon the Behavior during Reduction of the Pellets
As is well known, the metallurgical properties of pellets originating from pure magnetites, can be improved by adding silica. This should also be possible when starting from specularite-hematite ores. However, in this case, mechanical- physical properties will then be rather poor. The latter can be improved by basic additions, but as far as the metallurgical properties are concerned, the success varies. In the tests to be described below, it was the aim and objective to find out the interrelationships which might exist between the addition of lime and the metallurgical properties of the fired pellets.
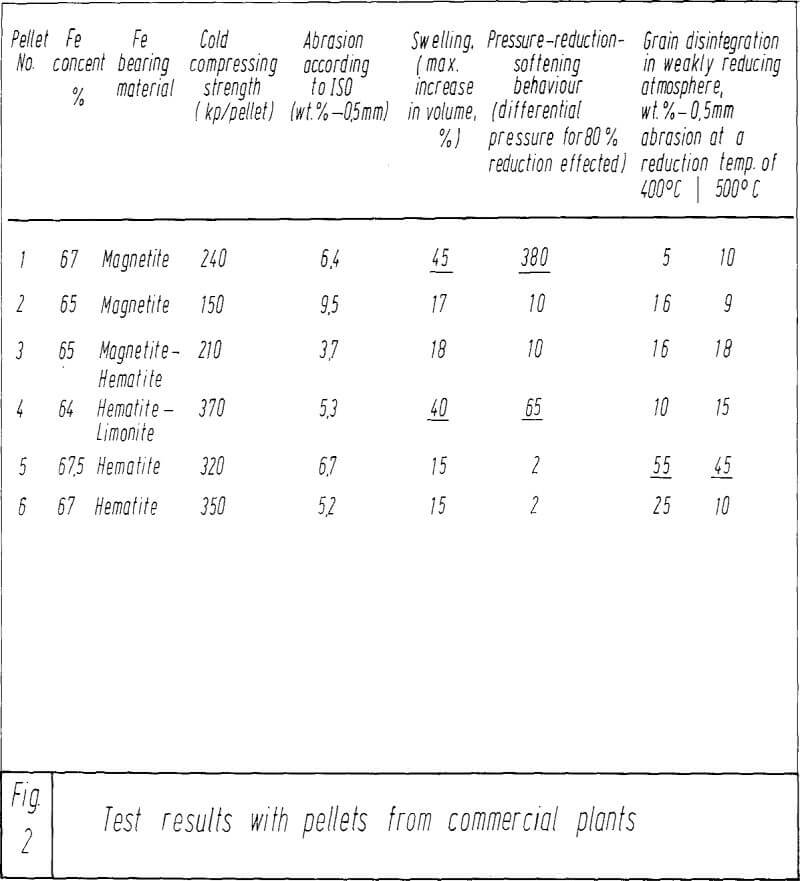
The results of the metallurgical tests executed at 1,000 deg. C. The results of the pressure-reduction-softening test are expressed in mm W.G. (millimeters water gauge) differential pressure, while in the right part, under B, the results of the swelling tests are expressed as the percentage of increase in volume, and that always as a function of the reduction effected. The SiO2 content was about 0.60%, while the CaO content was increased from 0.02 to 4.90 %. Pellet No. 1 represents the behavior of pellets made purely from the starting material, without any additions.
It is possible, on principle, to produce pellets of satisfactory metallurgical properties, even without corresponding CaO contents. This is true for pellets Nos. 4 and 15 whose resistance to gas passage is less than 10 mm, and which have experienced an increase in volume of about 20 % only. However, these pellets were not satisfactory, because their physical-mechanical properties, as, for instance, cold compression strength and abrasion, do not meet the requirements.
It is clearly to be seen that, theoretically, on the Fe2O3-SiO2 leg reactions between these two components do not start until very high temperatures have been reached. On the other hand, for the Fe2O3-CaO leg temperatures of reaction of about 1,200 deg. C are sufficient for the formation of new phases, particularly lime ferrites. Lime-silica phases are formed in the central part of the system at temperatures of from 1,200 deg. C to 1,300 deg. C. Here, one should make a reserve inasmuch as the phases illustrated in fig. 10, represent conditions of equilibrium and occur only when sufficient time is given to arrive at the equilibria. In the practice of pellet induration such a state is reached only nearly, but never completely.
Attempt to Interpret the Causes of Swelling, Loss in Strength and Grain Disintegration
Considering the test results on which this paper has been based, there must be still another factor which remarkably influences the swelling behavior of iron ore pellets to which lime has been added.
In this connection one must refer to the phase diagrams, in particular to the CaO-FeO-SiO2 phase diagram (fig 11). In the range from CaO·FeO·SiO2 to 2 FeO·FeO2 a Phase of solid solution (mixed crystal) exists which is characterized by a high dissolving power with regard to ferrous oxide. During the reduction this phase absorbs ferrous oxide which means that the melting point is lowered by some 100 deg. C. The temperature where this phase becomes fluid, may even be lower, probably less than 900 deg. C.
The higher the temperature of reduction, the more activity can be ascribed to such fluid solution. In order to reach saturation with ferrous oxide as quickly as possible, the fluid solution will make use of the ferrous oxide which is being offered. If this process occurs at the periphery of the crystallites, then it may happen that the slag bond which exists within the indurated pellet, gets lost.