Demand for manganese continues to grow, but essentially all manganese used in the United States is imported. Although large deposits of low-grade manganese minerals are located in this country, domestic manganese producers have been unable to compete with the foreign ore except when subsidized by the Government.
Desiring a degree of independence, both Government and industry have supported research to increase knowledge of manganese metallurgy. As a result, new methods have been developed for handling domestic low-grade manganiferous deposits, and old processes have been Improved. This Bureau of Mines report describes the use of sulfuric acid-ferrous sulfate solutions for extracting manganese from low-grade oxide ores, purification of the resulting solutions, and agglomeration of the residues to pellets for use as an iron furnace feed.
The first phase of this study was conducted to examine the feasibility of extracting manganese from Georgia umber ore by means of a three-stage continuous sulfuric acid-ferrous sulfate pickle liquor leaching process. The continuous cyclic process for treating the umber also was operated successfully using a synthetic leach solution of ferrous sulfate and sulfuric acid, simulating a mixture of ferrous sulfate pickle liquor from a steel pickling operation and sulfuric acid anolyte solution from a manganese electrolytic cell. For all systems tested, leaching efficiencies based on removal of manganese from ore ranged from 83 to 90 percent.
Also included in phase 1 was a brief study of residue agglomeration. The purpose was to show that pellets having both good green and excellent fired strength can be made readily from the leached umber residue. Acting as a binder, the filtered residue also was shown to blend well with several types of materials to form strong pellets; considering the residue’s iron content, large quantities of binder can be used without lowering the overall iron content of the pellets produced below acceptable levels.
Although the three-stage countercurrent decantation leaching system developed in the previous work provided good extraction of manganese from the Georgia umber, operational control of the balanced flow system was very sensitive. Even minor changes in feed rates and flows required immediate compensating changes at each control point throughout the system to maintain equilibrium. Thus, operating personnel and maintenance requirements for the system were high.
The second phase of the study, described herein, comprised research efforts to simplify the leaching operation and materials handling problems by converting the countercurrent leaching system to a single-stage cocurrent leaching system. Also included are the results of exploratory tests investigating the use of a centrifuge in place of settling tanks for separating leached residue from the different manganese leach solutions. Finally, the use of several different reagents for removing iron from the pregnant leach solutions is explored.
From a waste disposal viewpoint, successful application of this process by industry would diminish the costly waste disposal problem associated with sulfuric acid pickling of steel. With a practical outlet for the acid-ferrous sulfate waste, pickle liquors could be disposed of early while they still contain sufficient acid to leach the manganese oxide content in ores. This early disposal of the pickle liquor would prevent formation of basic iron sulfate gels, and the higher acid concentrations would increase the pickling rate.
Materials Used
Georgia Umber
Manganese deposits in northwest Georgia have a wide geographic distribution. The largest and most productive area is the Cartersville district.
The geology of the Cartersville deposits has been discussed by Kesler O’Neill and Wyndham state that the indicated reserves of umber in the district are large, about 10 million long tons, and that there is a reserve of 8 million long tons of manganese-bearing clays. According to Pierce, several cobaltiferous areas exist along with the ferruginous manganese deposits, with samples analyzing as much as 1.3 percent cobalt; however, the cobalt-bearing manganese material was low grade as to manganese. Another impurity of possible concern in these ores is a variable phosphorus content derived, according to Kesler, from a complex association of strengite and other iron phosphates. However, McCallie believed the main source of phosphorus to be apatite associated with the brown ores of the area.
The influence of time, temperature, pulp solids, and surface atmosphere on batch extraction of manganese from the low-grade manganiferous iron deposits of the Cartersville area are described in Bureau of Mines Report of Investigations 6452.
The ore used in this investigation was part of a 5-ton lot of umber obtained from the Cartersville area. As received, it consisted essentially of soft earthy chunks as large as 1.2 inches in diameter and contained 30 to 40 percent moisture. After air drying and grinding to pass a 20-mesh screen, the moisture content was about 20 percent. Additional drying occurring during storage further reduced the moisture content; therefore material requirements were based on material with all free moisture removed. The umber used for this study was essentially the same as that used in previous work, except for a slightly lower iron and manganese content. The ore analyzed 41.97 percent Fe, 4.38 percent Mn, 12.54 percent SiO2, and 13.20 percent loss on ignition at 1,000° C.
Leach Liquors
The leach liquors used in the different studies were sulfuric acid ferrous sulfate solutions simulating various possible combinations which might be discharged from a pickling operation.
Experimental Work
Cocurrent Extraction
The initial cocurrent leaching unit used for early tests consisted of a small mixing tank for blending ore from a table feeder with the leach solution. The slurry from the mixing tank passed by gravity to a 9-inch-diameter by 10-foot-deep thickener. Because of the small diameter of the thickener, it was necessary to construct a deep baffled center well to disperse the feed slurry. Although the system performed its functions as designed, the small feed and flow rates proved difficult to maintain for extended operations; therefore, a larger, less sensitive system was constructed.
The larger cocurrent system consisted of a standard-type ore conditioner for blending ore from a belt feeder with the leach solution. The slurry passed from the conditioner by gravity to a 2- by 10-foot thickener. Since the deep baffled center well worked well in the smaller unit, a similar center well was constructed for the 2-foot-diameter thickener. A diagram of the thickener reactor is shown in figure 1. Figure 2 shows both systems in operation.
Some maintenance of the liquid metering pumps to the conditioner was required during extended tests; otherwise, operation of the 2-foot-diameter by 10-foot cocurrent leaching system proved to be relatively simple compared with the countercurrent decantation leaching system. Although one operator was sufficient to make up solutions, recharge hoppers, collect products, and take control samples, a second operator was present to enhance safety by assisting and relieving the first operator and to analyze some of the samples. Operating parameters of a typical test are given in table 1.


Excepting mechanical problems, the system performed as expected. Some mechanical breakdowns occurred during the 15-day operation, causing short downtimes. These interruptions did not interfere seriously with the liquid-solids distribution in the cocurrent system, whereas similar interruptions in the previously reported countercurrent decantation leaching operation were quite serious.
Because of the influence of density in the settling of particles, it was considered important to examine the uniformity of the suspending liquid medium during leaching. Using a statistical method for comparing averages of several

products, it was shown that there was no significant difference in the mean specific gravity of the liquid phase throughout the thickener reactor. Extraction of manganese from the umber was about 87 percent.
A gradual decline in the solids content of the underflow discharge did occur during the 15 days of operation. Allowing time for removal of stored residue, data from the last 6 days of this decline were subjected to the Grear regression analysis to determine the rate of solids content decline.
A linear least squares fit to the data yielded the following equation:
y = 54.70 – 0.02725x,
where
y = percent solids,
and
x = time in hours.
A calculated mean solid content was equal to 52.8 percent solids.
The observed slow decline of solids content in the underflow continued as shown in the equation despite an attempted lowering of the solids removal rate. This suggested that the rate of solids compression in the thickener not the settling rate of the slowest falling particle, should control the maximum feed rate to this system.
Purification of Pregnant Leach Liquors
Purification of pregnant leach solutions is a problem common to all hydrometallurgical processes. In most instances iron is the major contaminant. The purpose of this phase of research was to investigate different methods for separating the relatively large amounts of iron from pregnant overflow solutions of the sulfuric acid-ferrous sulfate leach process, but because of time limitations, only three methods were tried; Chemical precipitation, ion exchange, and autoclave digestion.
If sufficient iron can be removed economically from the pregnant liquor to produce a manganese-enriched liquid for recycle to the system, production of a solution containing 30 gpl manganese or more could be obtained.
Chemical Precipitation
Chemicals considered for precipitation of iron were sodium hydroxide, soda ash, milk of lime, ammonia, and a mixture of ammonia and carbon dioxide. Sodium hydroxide was chosen for the initial study of variables affecting removal of ferric iron from the pregnant overflow leach liquor because of the stability of reaction products and ease of handling. Variables investigated included concentration of the neutralizing agent, time, temperature, rate of addition, and degree of agitation.
The procedure in most of the 30 tests completed consisted of placing 2 liters of pregnant overflow leach solution containing 38.4 grams of ferric iron, 0.6 gram of ferrous iron, and 17.0 grams of manganese in a 4-liter stainless steel container. The solution was agitated and brought to test temperature, and a fixed quantity of sodium hydroxide was then added. The pH of the resulting slurry was 4.7 to optimize precipitation of iron and prevent precipitation of manganese. The iron-bearing precipitate produced by the reaction was separated from the solution by filtration, washed, dried, and weighed, Both the filtrate and residue solids were analyzed for iron and manganese so that a material balance could be made, Selected data of the tests are given in tables 2, 3, 4, and 5.
Examination of the data in tables 2,, 3, 4, and 5 revealed that precipitates formed at 70° C and above filtered much more freely than those formed at lower temperatures. In all tests, the quantity of sodium hydroxide used to bring the pH of the solutions to 4.7 was only about 75 percent of the stoichiometric amount needed to convert all the iron to the corresponding hydroxide. Precipitation of nearly all of the iron with less than the required amount of reagent calculated to completely precipitate the iron as the hydroxide and the iron analysis of the filter cake suggest that the iron probably is largely present as the basic sulfate of iron in the precipitate. This material in equilibrium with the hydroxides of iron may explain the thixotropic properties encountered with this material.
The tests also showed that for sodium hydroxide, the temperature, rate of addition or time, normality of the solution added, and degree of agitation had little influence on the amount of iron or manganese deposited, but the more concentrated precipitating agent permitted a higher concentration of manganese in the final product (see test 4 of table 4). Analyses of the different wash solutions indicate that washing of the precipitated iron to recover additional manganese values or their recycle to the system must be included in the pregnant liquor purification circuit.
Because of the limited time remaining for test purposes, a basic array of 25 tests for five variables was designed using a modified “Latin Squares” technique which has been described previously for fewer factors. These data were used to confirm observations made during the sodium hydroxide test work and to compare other chemicals under similar operating conditions. The variables tested for each of the chemicals were again contact time, temperature, concentration, and the amount used. It was understood that interaction among variables would distort the results; however, for these initial comparisons, the degree of interaction was assumed negligible as suggested by Gore. Comparison of results was based on recovery of manganese in the filtrate, deposition of iron, and the time required to collect 500 ml of filtrate after reaction. Test conditions used for the 25 tests and the test results are given in table 6.

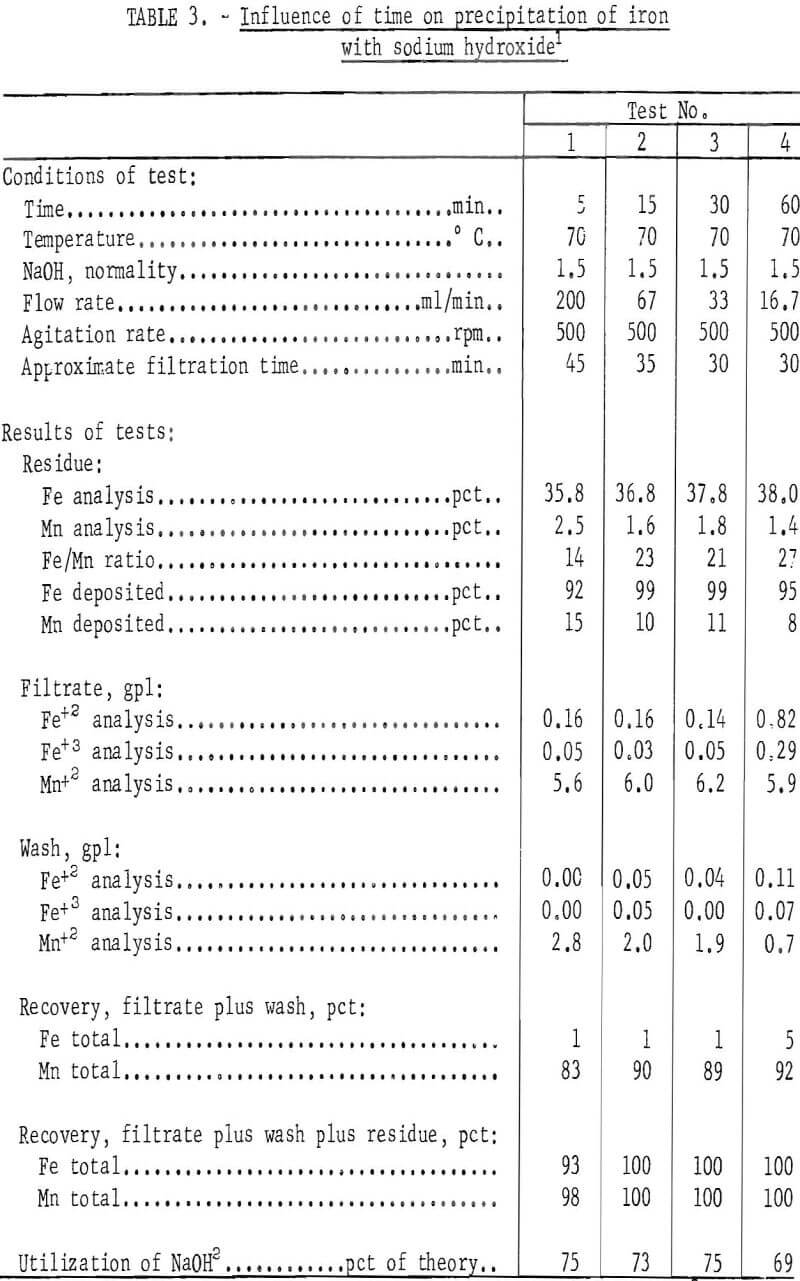



Results of 25 tests of table 6 showed that when using as little as 70 percent of the different reagents required to convert the iron to a ferric hydroxide, 88 percent or more of the iron was deposited. Increasing the amount of reagent above 70 percent of the theoretical amounts required to precipitate all of the iron as ferric hydroxide appeared to increase the amount of iron precipitated, but also adversely precipitated more manganese.
Except for calcium hydroxide, the relative filtration time decreased rapidly with increased temperature; at the lower temperature, the pulps became more viscous and difficult to filter. While ammonia, or ammonia and carbon dioxide, appeared best suited for recovery of manganese as manganese sulfate in the filtrate, iron precipitated with calcium hydroxide filtered best. There was very little difference in the filtration time among tests made with calcium hydroxide.
Taken together, the test results suggest that only about 70 percent of the calculated amount of the different reagents was sufficient to precipitate most of the iron from the pregnant leach solution. Except for calcium hydroxide, temperatures of 45° C or higher should be used to avoid formation of a gelatinous precipitate which was difficult to filter. Although calcium hydroxide did not form the gelatinous precipitate even at room temperature, considerable manganese sulfate was lost by entrainment in the filter cake. Good mixing of the reactants to avoid local high concentrations of base reduced manganese entrainment slightly. Subsequent tests not shown here confirmed this observation.
Ion Exchange
In 1954, Robert Kunin applied ion exchange to the recovery of sulfuric acid from waste pickle liquors of the steel industry. Although ion exchange resins have been used extensively to separate the more valuable and rare metals from dilute solutions, application of the method to separating relatively abundant low-value metals such as manganese and iron has been limited largely to analytical procedures.
Concentration and beneficiation of manganese with respect to iron in pregnant solutions recovered after leaching the Georgia umber was considered worthy of investigation. The treatments were restricted to a simple loading and stripping operation. Variables tested were flow rates and concentration at various manganese-iron ratios. Both resin-in-pulp (RIP) and column systems were tried using a cation resin consisting of 96 percent styrene and 4 percent divinylbenzene.
The exploratory tests showed that at equilibrium, the manganese-iron ratio on the resin was essentially independent of the initial feed concentration and the flow rate. Also at equilibrium, the manganese-iron ratio on the column was about 2.3 times that of the feed solution. When the resin contact was less than that required to establish equilibrium conditions, a slight decrease in the manganese-iron ratio occurred.
Because of the resin’s selectivity for manganese, several multiple loading and stripping tests were made. These tests indicated that five cycles would be needed to increase the manganese-iron ratio from 0.15 in the influent to 11.5 in the eluate. This suggests that separation of manganese and iron could be accomplished, provided sufficient loading and stripping operations were attempted, but that handling costs of such a system would be high.
Autoclave Digestion
Because of the improved filtration characteristics of reaction products produced during chemical precipitation at temperatures above 70° C, and the observations of Katsuri that gelatinous iron hydroxide in an autoclave under about 5 atmospheres pressure changes to a rouge like powder, the influence of temperatures above 100° C was investigated, using an autoclave for leaching the ore while at the same time depositing much of the iron from solution. The results of the extraction tests are given in table 7.

A possible explanation for the increased extraction of manganese at elevated temperature and pressure is that the additional pressure forces liquid farther into gas-filled capillary openings in the ore particles, thus exposing additional surface area to the leaching action of the ferrous sulfate solution; also, higher temperatures lower the viscosity of the liquid medium, thereby permitting even more effective penetration of capillary opening.
Deposition of ferric iron from the leach solution resulting from autoclave treatment was nearly complete; however, some unreacted ferrous iron remained in solution. Other autoclaving tests showed that the rougelike-powder iron precipitate can be removed from the manganese-bearing solution by filtration after cooling; alternatively, taking advantage of the inverse solubility of manganese sulfate, the autoclaved liquid can be filtered at the higher temperature and pressure. These tests used both synthetic solutions containing manganese sulfate, ferrous sulfate, ferric sulfate, and sulfuric acid with or without ammonium sulfate, and leach solutions from the earlier countercurrent decantation leach operations. Thus, excess liquid containing undesirable impurities can be separated from the manganese sulfate and iron precipitate, and both the manganese sulfate and the precipitated iron collected on the filter; then by water leaching the filter cake, a manganese concentrate can be readily separated from the iron residue. Precipitates formed at elevated temperatures and pressures ranged in color from pale yellow at about 110° C to yellow-brown at 130° C; at higher temperatures, the precipitates rapidly assumed the dark red color of hematite.
Air Oxidation
An exploratory test was made to see if air could be used to precipitate the ferrous and ferric ions from the pregnant leach liquor. The expected reactions were-

The test consisted of introducing air by means of a high-speed aeration cell into pregnant leach solution containing a small quantity of frother to provide surface contact area. The cell was equipped with an overflow pipe extending well above the surface of the liquid phase. The froth produced above the liquid surface was evacuated from the system through the overflow pipe. After the froth became clear of solids, an analysis of the remaining liquid phase indicated <0.02 gpl Fe+², <0.02 gpl Fe+³, <0.02 gpl Fe total, and 6.2 gpl Mn+². Additional work to develop the parameters of this approach to iron removal is recommended.
Other Exploratory Testing
An exploratory test deemed worthy of mention because of its recycle potential involved the use of sulfur dioxide gas for in situ regeneration of ferrous sulfate from the leach reaction products, ferric sulfate and basic iron sulfates. The brief test indicated that approximately 2.3 pounds of sulfur dioxide was used to regenerate sufficient ferrous sulfate to extract 1 pound of manganese. Sulfur dioxide also proved beneficial for retarding gel formation during leaching.
Centrifuge Tests
Acceptance of the centrifuge is increasing despite its high initial and maintenance costs because of its compact size and high separation capacity. The development of improved designs and new corrosion- and wear-resistant materials of construction also contribute to its increasing popularity.
Because of the centrifuge’s exceptional ability to handle fine particles which are difficult to settle, it was considered as a possible substitute for thickeners. A short series of tests was designed to demonstrate this capability, using a laboratory-size solid-bowl centrifuge.
Initial shakedown tests were made to establish a suitable operating feed procedure to the centrifuge. The feed procedure established was as follows: The overflow slurries produced by mixing air-dried ore with leach solutions containing 50, 90, and 130 gpl ferrous sulfate and different quantities of sulfuric acid in a conditioner were fed to the centrifuge through a vibrating screen, which removed oversize particles. Sufficient centrifuge samples were taken to enable collection of the data shown in table 8.
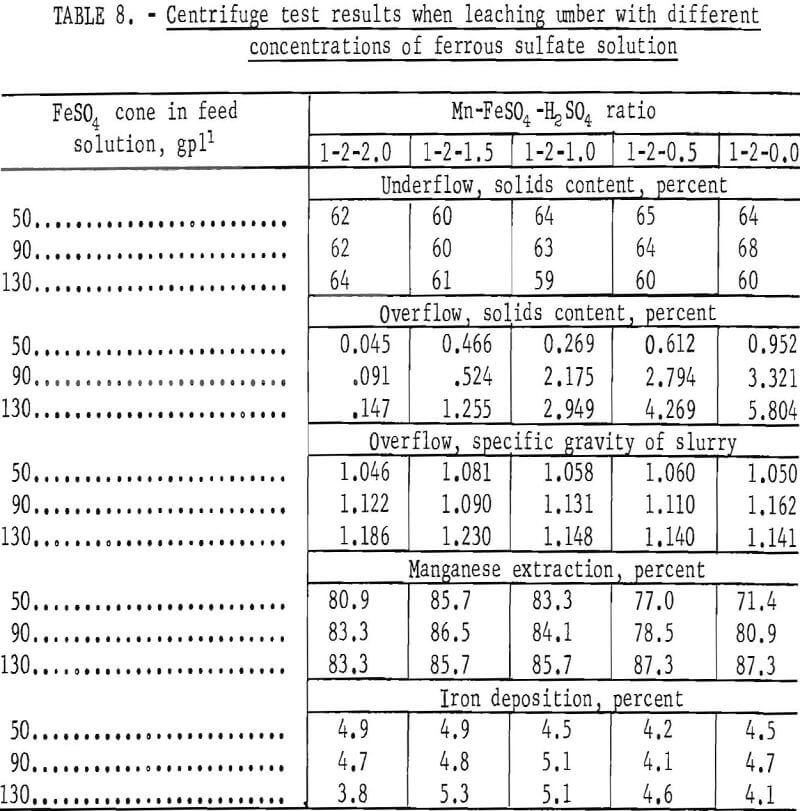
The data in table 8 indicate that the capabilities of the centrifuge surpass those of the thickener in several areas. Not only could the centrifuge handle concentrated leach slurries which the thickeners could not, but the solids content of the underflow fraction contained appreciably less moisture; that is, approximately the same as values obtained by filtration. The small quantity of solids in the overflow can be easily eliminated by either reducing the feed rate slightly, recentrifuging, or filtering. Extraction of manganese and deposition of iron was about the same as for thickeners.
Planned tests to operate at higher ferrous sulfate concentrations were abandoned because of apparent increasingly corrosive conditions which might damage the unit. However, three tests using a synthetic anolyte solution as the leaching agent also were successfully completed without difficulty.
Summary and Conclusions
The ferrous sulfate-sulfuric acid leach process for extracting manganese is a versatile and technically feasible process which can be adapted to fit a variety of circumstances. Figure 3 shows an example that could be used. The process flowsheet may be altered somewhat depending on the characteristics and composition of the ore to be leached, the end products produced, and the type of equipment used.

Leaching of umber occurs rapidly, as shown by centrifuge tests wherein the ore was mixed with acid-ferrous sulfate solution in a conditioner and the solids were separated immediately thereafter by centrifuging the conditioner overflow. Where space, is a factor, centrifuging has a distinct advantage over settling and thickening. Operating costs and low profit margin with low-grade ores, however, favor the settling and thickening approach to liquid-solids separation. Long contact of liquids and solids during settling and thickening favors slightly better extraction of the manganese metal values. The cocurrent extraction system was easy to operate and control, and it was essentially trouble-free. This is in contrast to the sensitively balanced staged countercurrent system used in the earlier work.
The research presented demonstrates the ease with which manganese can be extracted from oxide-bearing ores. The extracted manganese then can be separated from iron-bearing residue by settling and thickening or by centrifuging the pregnant leach slurry. The manganese concentration of the product liquor can be adjusted over a wide range by recycling a portion of the product after regeneration with SO2 or after removal of iron by one of several different methods available. As an alternative one also can concentrate the manganese by autoclave treatment using the inverse solubility property of manganese. Manganese then can be isolated as metallic manganese by electrolysis, as manganese sulfate, or as manganite by chemical precipitation. The possibility of recovering associated metal values such as iron may be a potential source of credit for the process. Choice of the different possible combinations would be based on the local situation and other economic factors which were not studied in this research effort.
Despite minimal costs for chemicals and allowing credits for byproduct iron, the low manganese content of Georgia umber does not suggest a favorable picture for application of the process to the Cartersville deposit. If the manganese content of some other ore were sufficiently large, or if the umber could be physically concentrated with respect to manganese, this technically feasible process does have merit and should be considered.
