Study of light adsorption, reaction rates, electrophoresis, particle packing, and corrosion is hindered by lack of a suitable method of preparing small amounts of closely sized materials in the micron and submicron range. Most fractionation methods depend on some form of particle movement through a viscous media in which velocity of a particle, relative to the media, is controlled by particle size. Fractionation by settling at particle sizes of 0.1 to 5.0 is best by many difficulties, chiefly:
- Thermal currents and Brownian movement result in disordered particle motions at velocities comparable to settling velocities of the particles.
- The resolution obtained with ordinary light microscope techniques is not sufficient for satisfactory analysis of results of a separating operation.
- Flocculation prevents particles from acting as individuals and the settling characteristics of the particles are altered.
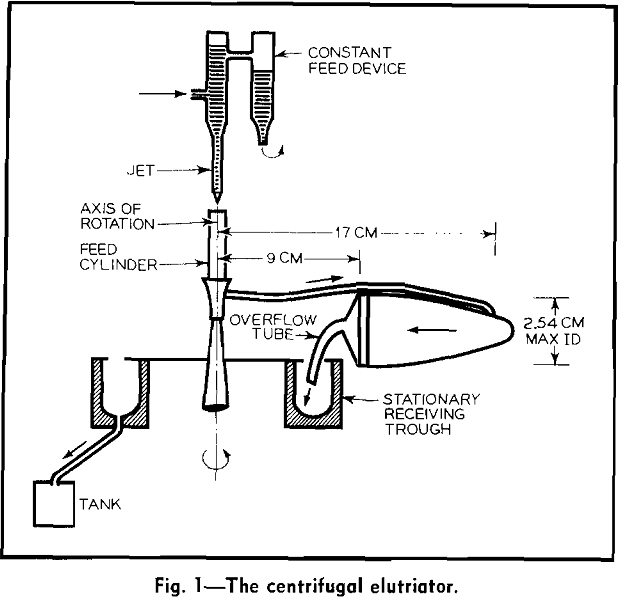
A dilute water suspension of the particles to be fractionated enters the feed cylinder from a controlled flow jet. The suspension flows through the connecting tube and the centrifuge bottle under the action of a static head and a centrifugal force. Flow of water through the bottle carries particles finer than a critical size over the lip of the bottle into the stationary receiving trough. Coarser particles remain in the centrifuge bottle. 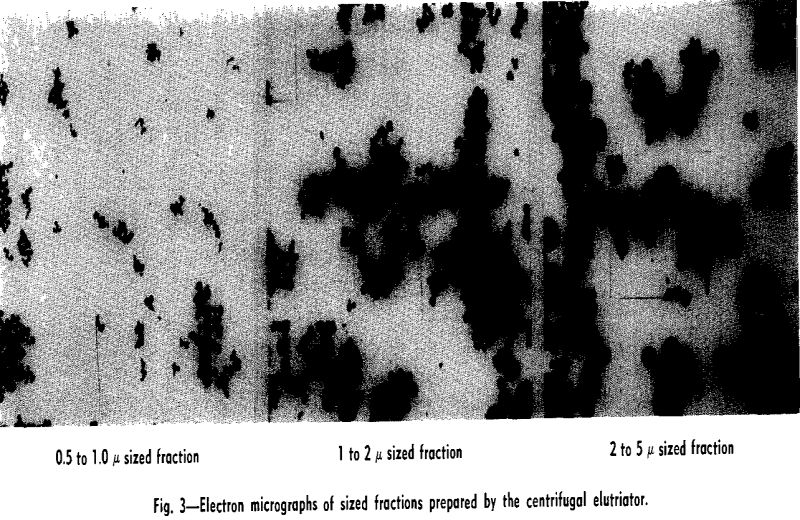
Stokes’ law is as follows:
Upward viscous flow force on particle = Downward force of gravity on particle
6πrηv = 4/3πr³(pspl)g…………………………………………….[1]
where η = coefficient of viscosity of fluid, v = critical fluid velocity, r = particle radius, ps = density of particle, pl = density of fluid, and g = acceleration of gravity.
6πrηv = 4/3πr³ (ps — pl)xω²……………………………………………………[2]
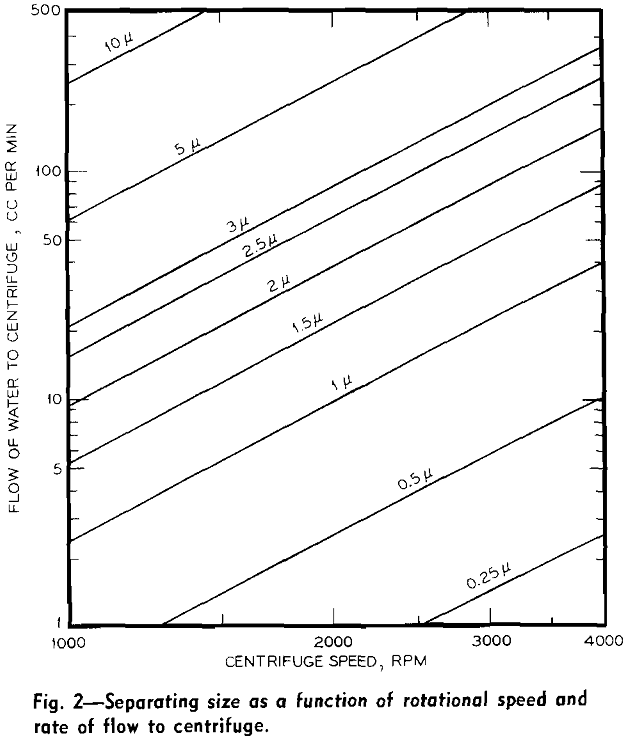
ds = √18 Vη/Ax(2πN)² (ps – pl)…………………………………………………[3]
where ds = separating size in centimeters, A = cross-sectional area of centrifuge bottle at point of discharge in cm², V = volumetric rate of flow in cm³ per sec, x = distance from center of rotation to point of discharge of the centrifuge in centimeters, and N = rotational speed in revolutions per second.
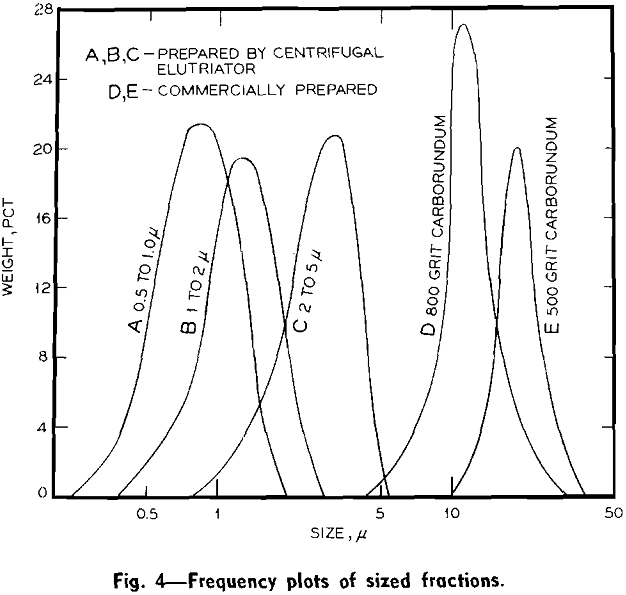
Experimental Results: Size Fractionations: Tests were run with the centrifugal elutriator to obtain sized fractions between 5 and 2 µ, between 2 and 1 µ, and between 1 and 0.5 µ. To obtain these fractions a size separation was made of the feed material at the maximum desired size, taking the overflow for feed to the elutriator and making a size separation
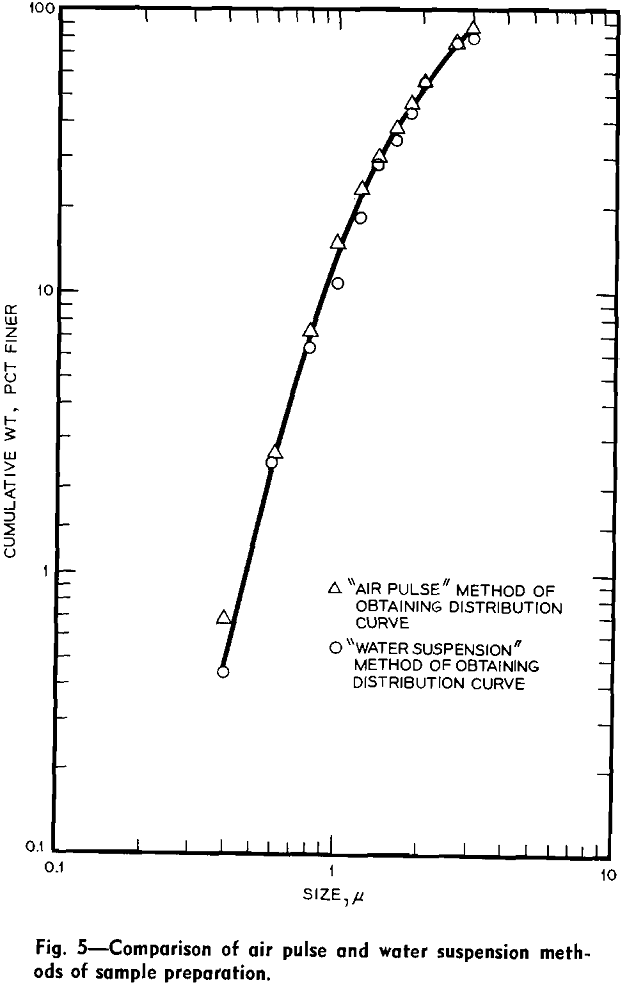
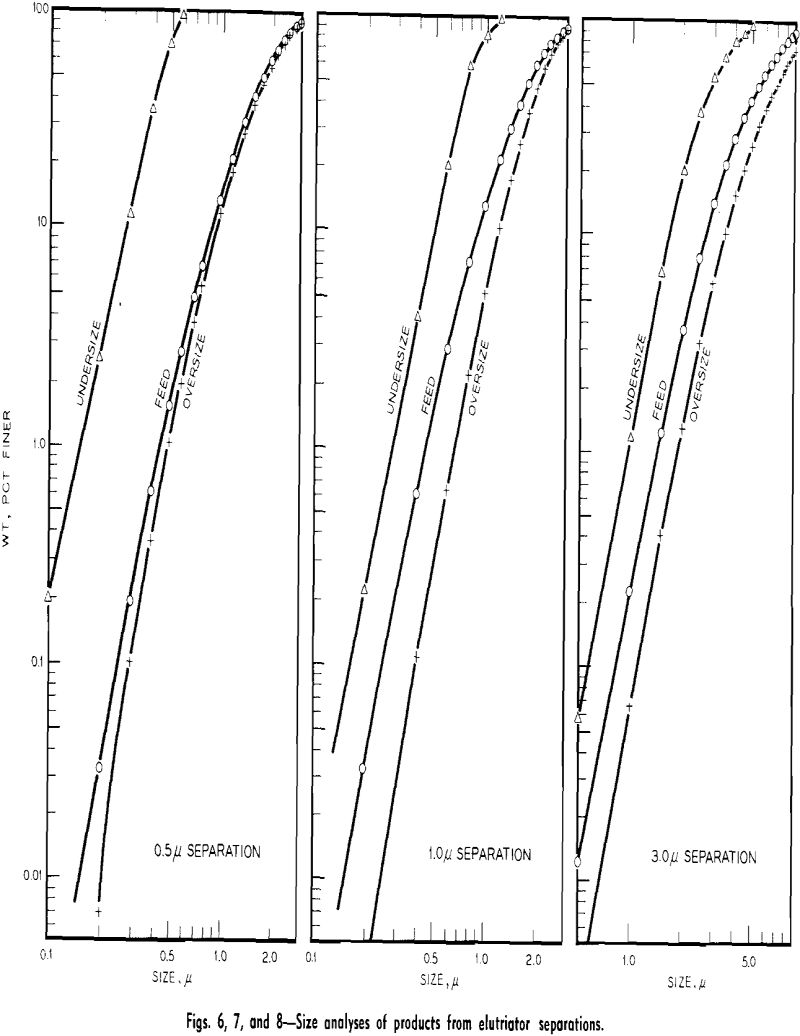
at the minimum desired size. The material remaining in the centrifuge bottle from the last separation was the desired size fraction.
Unfortunately the amounts of material recovered in the fractionations by the centrifugal elutriator were small. In general, for a test of 1 g of material in 1 liter of water, between 20 and 50 mg of fractionated material would be recovered.
Size Separations: By means of sedimentation-decantation of portions of the original material prepared in the flame spheroidizer, a sample containing spherical glass particles less than 5 µ and a sample containing glass particles less than 10 µ were prepared. These two samples were used as feed to the centrifugal elutriator for various size separation tests.

