Arsenic is produced in the United States as a byproduct from base metal ores. Although this production supplies only about one-half of the national demand, arsenic is generally not recovered but is treated as a troublesome impurity. Its complete recovery in domestic metallurgical plants could nearly supply the country’s arsenic needs, but complicated and inefficient technology coupled with a fluctuating market has made recovery unattractive. Thus a potential resource has become a toxic waste diposal problem.
In keeping with its goal of maximizing recovery of metals and minerals from domestic resources, the Bureau of Mines, U.S. Department of the Interior, is investigating the chemistry of accessory metals during base metal processing. This report is intended to provide knowledge about the disposition of minor elements during smelting of base metal ores.
Copper ores are the largest potential domestic source of arsenic. During copper smelting, arsenic is diluted and emitted in the gas and slag streams, necessitating special collection and handling procedures.
Roasting, the first step in most copper smelters, is relatively inefficient as an arsenic removal process. Multiple-hearth roasters generally remove about 20 percent of the arsenic contained in the feed. Experimental fluidized-bed roasters have been shown to remove from 10 to nearly 100 pct of the incoming arsenic, depending upon the heating schedule, atmosphere, feed composition, and number of beds in tandem. The primary functions of roasting are drying and heating the incoming feed and, in some instances, sulfur removal. Arsenic, antimony, bismuth, lead, and other accessory elements are vaporized in subsequent operations.
Accessory metals, although valuable resources, present possible environmental concern in the smelting of base metals. The Bureau of Mines, U. S. Department of the Interior, has undertaken research to define the chemistry involving these metals. Arsenic in copper smelting is the first to be investigated.
Roasting, the first step in many copper smelters, was chosen for initial investigation because of the potential for arsenic removal in this primary process. Simple, static-bed, vaporization experiments were devised to obtain the data in this study. Ancillary transpiration and mass spectrometry were also used to delineate conditions necessary for arsenic volatilization from a roaster feed. For nearly complete arsenic removal, a temperature of 650°- 700° C, a reducing atmosphere including carbon monoxide, and a source of sulfur were found to be essential.
If essentially all of the arsenic could be volatilized in the roasting process, arsenic collection would be simplified and could be made more efficient. Considering the relatively high vapor pressures of arsenic, arsenic sulfide, and arsenic oxide it appears that arsenic would be removed at roasting temperatures in either oxidizing, reducing, or neutral atmospheres. However, this is not the case.
The literature is somewhat ambiguous on factors affecting arsenic volatilization during sulfide roasting, A recent paper relates arsenic elimination to operating conditions in commercial roasters and concludes that the volatilization of arsenic depends upon temperature, residence time, and atmosphere.
Considering their view of roaster operations and other reported experimental work, four factors emerge as important influences on arsenic during roasting; temperature, rate of heating, oxygen pressure, and the presence of sulfur.
The ultimate temperature is of primary importance and must be high enough to break down the original sulfide minerals and to insure adequate vapor pressure of the volatile species. Arsenic removal from pyrite ores has been reported to occur at 500° to 900° C. The controlling step in this removal appears to be the decomposition of arsenopyrite, which begins at 500° C. Enargite, a copper-arsenic sulfide, was found to thermally decompose above 600° C, and a copper concentrate containing enargite lost arsenic above this temperature. In general, the lower temperature limitation is dictated by initial mineral breakdown. On the other hand, too high an ultimate temperature was found to cause sintering and agglomeration of pyrites and this in turn retarded arsenic elimination.
The heating schedule, particularly initial heating rates, reportedly influences arsenic volatilization. For example, when roasting gold ores prior to cyanide extraction, researchers have in the past advocated both slow and fast heating rates for arsenic removal. The interpretation of these heating recommendations is complex, involving the evolution and partial pressure of SO2. Rapid heating, which generates higher partial pressures of SO2, limits the free oxygen available for further oxidation of the components. Under these milder oxidizing conditions, formation of nonvolatile arsenates is assumed to diminish. On the other hand, it has been suggested that during the rapid exothermic oxidation of sulfur to SO2, the temperature rises, causing local overheating and fusing of individual particles, which in turn destroys porosity and hinders vapor escape.
Oxygen partial pressure is generally accepted in the literature as an important factor in arsenic elimination. One author describes “locking in” of arsenic when roasting with excess air. This process is believed to be the formation of pentavalent arsenic, the less volatile oxide, or arsenates. To prevent such oxidation, it has been suggested that a reducing atmosphere be used, or that a more labile material be present to combine with excess oxygen. Coal and sulfur have been mentioned as “activating agents” for removing arsenic during roasting, presumably because of their ability to combine with oxygen. Above 600° C, oxidation by carbon dioxide may even be a factor, and it has been suggested that the partial pressure of this gas be kept low. Because roasting is a dynamic process, oxidation reactions occurring in a roaster may be more important than thermodynamic equilibria in determining the final products.
Sulfur is considered to be an important ingredient for arsenic removal. Earlier reports stated that arsenic sulfide was the volatile species and implied that oxidation hindered arsenic removal. More recently thermodynamic calculations and experimental work suggested that the importance of sulfur lies in the fact that it prevents the formation of iron oxides. Trivalent iron oxide is credited with enhancing the retention of arsenic by the formation of iron arsenate. This hypothesis could be extended to other metals, such as copper.
Because of the conflicting views of arsenic behavior and the need for a better understanding of arsenic volatilization during roasting, we have conducted tests designed to monitor arsenic distribution within beds of copper smelter feed materials under various heating rates, ultimate temperatures, times, and solid and atmosphere compositions. The results of these experiments are used to arrive at criteria for the elimination of arsenic during heating of these materials.
Experimental Work
Materials
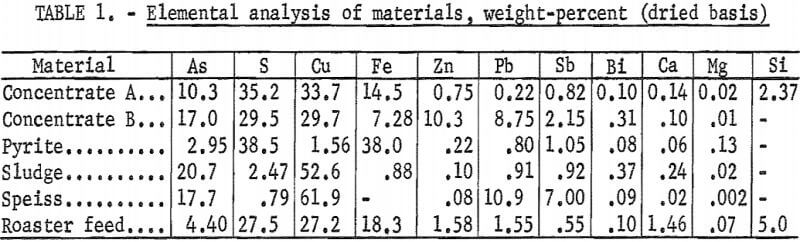
Five individual copper-bearing materials and a composite roaster feed were used in the experimental work. Table 1 lists the elemental analysis of these components. Concentrates A and B are from typical mixed sulfide ores but have higher than usual arsenic contents. Tennantite, energite, chalcopyrite, and pyrite have been identified in these materials. The sample referred to as pyrite is primarily pyrite with some tetrahedrite and arsenopyrite. However, electron microprobe examination of the concentrates and the pyrite showed that the copper, iron, sulfur, arsenic, zinc, lead, and antimony were intimately associated in various combinations not necessarily corresponding to the minerals mentioned. The sludge sample was recovered from electrorefining operations. Larger soft copper particles in the sludge were removed by screening because they could not be ground to a finer size. According to electron micrographs, the copper, zinc, and antimony are about evenly and completely distributed through the sludge particles, whereas the arsenic and lead tend to be associated. The speiss is a dense, hard smelter byproduct. Generally, the copper is not associated with the other metals; arsenic and antimony are found with lead, but not with each other; and the zinc and iron are distributed evenly throughout the speiss. The roaster feed contains the same high-arsenic-content materials in the following proportions; in percent;

Other copper concentrates, containing minor amounts of arsenic, make up approximately the remaining three-fourths of the roaster feed. Hie arsenic concentration is this composite is high when compared to average roaster feeds. Accurate elemental analyses and attempts to identify compounds dictated the use of the high-level arsenic materials.
Individual materials to be pelletized were dried, ground to minus 100 mesh, mixed with 4 pct kaolin, wet-rolled into pellets, sized to minus 16 plus 20 mesh, and dried. These pellets provided a bed that retained its size during heating, had a uniform composition with adequate and consistent porosity, and reacted uniformly within the individual pellets. Compositional changes were noted with respect to bed depth. Compositional uniformity, radially in the bed and radially within the individual pellets, was verified by electron microprobe studies.
 Experimental Procedure
Experimental Procedure
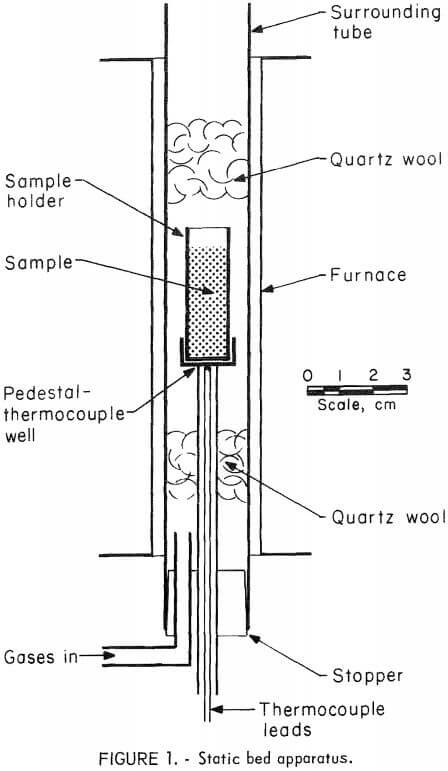
Figure 1 shows the apparatus used for the static bed experiments. Pellets were loaded to a depth of 3 cm in a flat-bottomed silica tube 11 mm in inside diameter and 4 cm high. A loaded tube was positioned on a thermowell-supported pedestal in the center of a 25-mm-diameter tube. Insulating quartz wool tufts were placed a few centimeters above and below the sample, and a rubber stopper with an inlet glass tube closed the bottom end of the tube. Gas flows were adjusted upward through this vertical tube and around the sample to 150 cm³/min.
Heating was accomplished by a 30-cm-long split-tube furnace. Three heating rates were used: the maximum achievable (from room temperature to 700° C in 20± 5 min), and lesser rates of approximately one-haIf and one-fifth of this maximum. Once at the desired temperature, the sample was held to ±10° C. Cooling was achieved by removing the entire apparatus from the furnace. When cool, the pellets were carefully removed in horizontal layers with a special scoop. Each layer (usually 0.5 cm thick) was analyzed for arsenic and sulfur content.
These experiments are a step in simulating roaster operation and reactions within a bed, and were not intended to determine the effects of rabbling and falling.
Results and Discussion
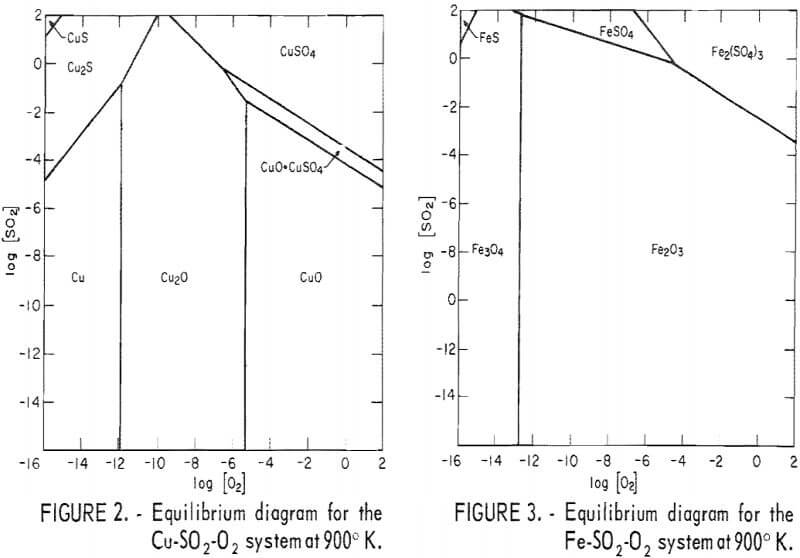
To obtain a basis for the interactions between the five major elements involved in roasting (Cu, Fe, As, S, and O), thermodynamic data were compiled. Although the literature does not contain complete thermodynamic values for those compounds involving arsenic in copper roasting, the available data were used to construct figures 2-4. Figures 2 and 3 summarize the effects of oxygen and sulfur dioxide on the equilibrium state of copper and iron at 900° K; they are based on thermodynamic data to be published by the Bureau of Mines and authored by E. G. King, A. D. Mah, and L. B. Pankratz. Figure 4 shows pertinent equilibrium reactions involving arsenic at this temperature. Data for these reactions come from reference 11 or were estimated by the authors. Although the accuracy of these later data may be less reliable, they indicate the range of conditions associated with the volatilization of arsenic. Of particular interest is the scale at the top of figure 4, which indicates the relatively low ratio of carbon monoxide to carbon dioxide necessary for the formation of the volatile arsenic trioxide.
Together, these three figures show the major compounds expected at equilibrium and at the ultimate roasting temperature. At lower temperatures, the higher valence, nonvolatile arsenic compounds are stable at lower oxygen pressures. Therefore, other conditions being constant, these compounds could be made to yield volatile arsenic trioxide simply by heating (that is, higher temperatures and lower oxygen pressures would cause decomposition to volatile AS4O6).
 As a first step in the experimental work, preliminary thermogravimetric and differential thermal analysis tests were made with the individual materials listed in table 1. Because the materials themselves were made up of several minerals, and even the identified minerals contained substituted metals and sulfides in solid solution, the results were too complex to be of use.
As a first step in the experimental work, preliminary thermogravimetric and differential thermal analysis tests were made with the individual materials listed in table 1. Because the materials themselves were made up of several minerals, and even the identified minerals contained substituted metals and sulfides in solid solution, the results were too complex to be of use.
A time-of-flight mass spectrometer was used to identify the gaseous constituents emitted from heated roaster charge. These analyses showed AS4 to be the predominate vapor molecule when the heating occured in argon, carbon monoxide, or carbon dioxide. As4O6 predominated when air was present. As3S3 was the only other arsenic-containing vapor constituent found. It appeared at a lower concentration in all cases, and was much lower with air present.
To evaluate the thermodynamic data and mass-spectrometry results, transpiration tests were made in a horizontal tube using 5- to 10-gram samples of unpelletized material in an open boat. From such experiments both the residue and some of the condensed vapors could be collected and analyzed. However, these experiments proved unsatisfactory because of product segregation by both depth and length in the bed. Even with a rapid gas flow and with the solid sample spread quite thinly, segregation was observed in the residue.
It became evident that an understanding of the mechanism of arsenic loss could be obtained from investigating such segregation in a narrower, deeper sample. Therefore the pellet bed experiments were developed. In these experiments , reactions between escaping vapors, the surrounding atmosphere, and the solids produced composition changes, which were uniform with respect to sample diameter but which varied with sample depth. This was easily observed in experiments with air; they produced a bed of nearly perfect horizontal layers of different colored reaction products. Other atmospheres did not produce such vividly colored products. Microscopic examination of pellet cross sections from these same experiments showed uniform reaction with respect to pellet diameter. Relying on this radial uniformity, the quantitative results from the experiments were expressed as residual arsenic concentration profiles along the depth of the pellet beds. The conditions of the tests are given in tables 2 through 6.
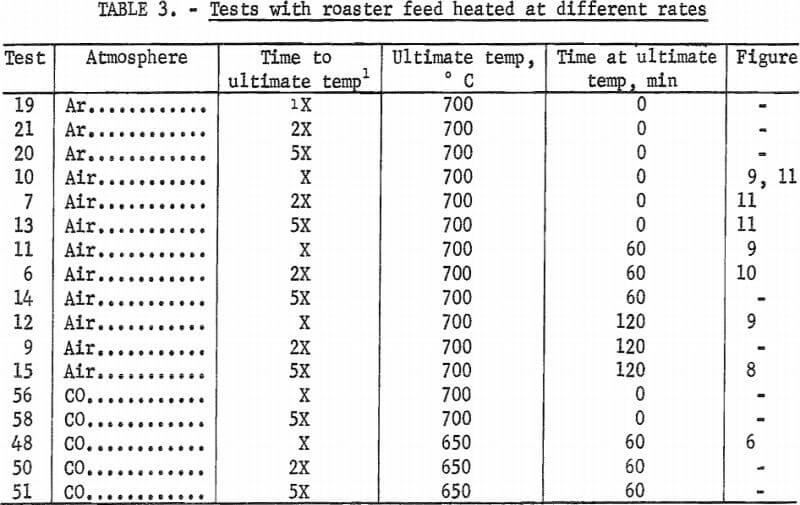
Figures 5, 6, and 7 give comparisons of arsenic and sulfur profiles in roaster feed heated in neutral (Ar), reducing (CO), and oxidizing (air) atmospheres, respectively, at three temperatures. In the neutral atmosphere, arsenic vaporization appears to be controlled by vapor diffusion upward through the bed. The partial pressure of the vapor is dependent on temperature.
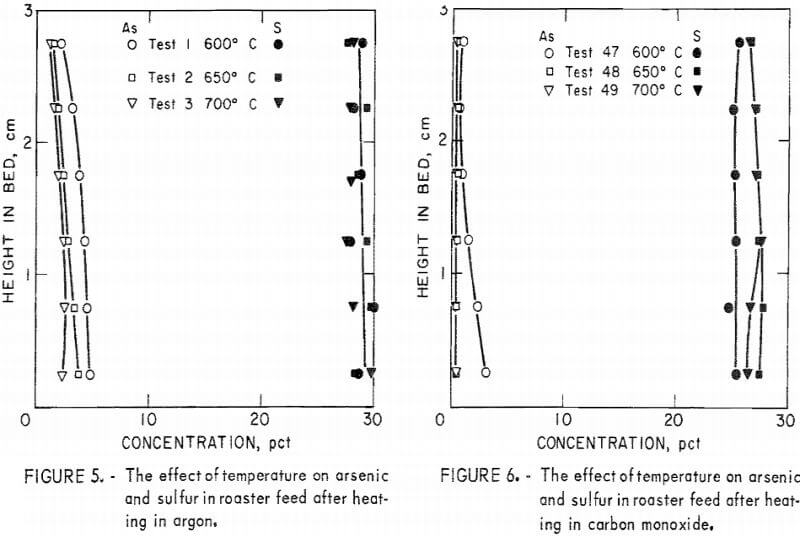
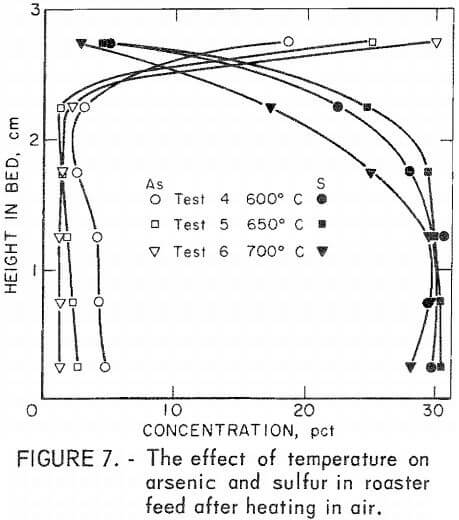 Figure 6 shows that a similar situation occurs in carbon monoxide but arsenic elimination is improved, most likely owing to reduction of arsenate-foaming oxides present in the original material. With both of these atmospheres, sulfur is little affected. Such is not the case in air. Figure 7 shows that sulfur concentration decreases In the upper part of the bed where it can readily react with oxygen and be eliminated as SO2. Arsenic, on the other hand, is concentrated in the oxidizing region at the top of the bed.
Figure 6 shows that a similar situation occurs in carbon monoxide but arsenic elimination is improved, most likely owing to reduction of arsenate-foaming oxides present in the original material. With both of these atmospheres, sulfur is little affected. Such is not the case in air. Figure 7 shows that sulfur concentration decreases In the upper part of the bed where it can readily react with oxygen and be eliminated as SO2. Arsenic, on the other hand, is concentrated in the oxidizing region at the top of the bed.
X-ray diffraction analysis of residue layers from a similar experiment, test 15 (fig. 8), revealed that the primary identifiable constituents of the upper bed are copper sulfate and iron oxide. Although arsenic is a major element in this area, its compounds were not detected by X-ray diffraction which requires well defined crystal structures. The slowest heating rate was used in this test and the ultimate temperature was maintained for 2 hours. Elemental analyses of the narrower layers at the top of the bed in this test better define the arsenic and sulfur distribution. Surprisingly, the arsenic level was found to be nearly nine times greater in a layer near the top of the bed than it had been in the original material, while lower in the bed no arsenic was detected. Sulfur decreased to a minimum near the top of the bed, but increased on or near the surface as sulfate.
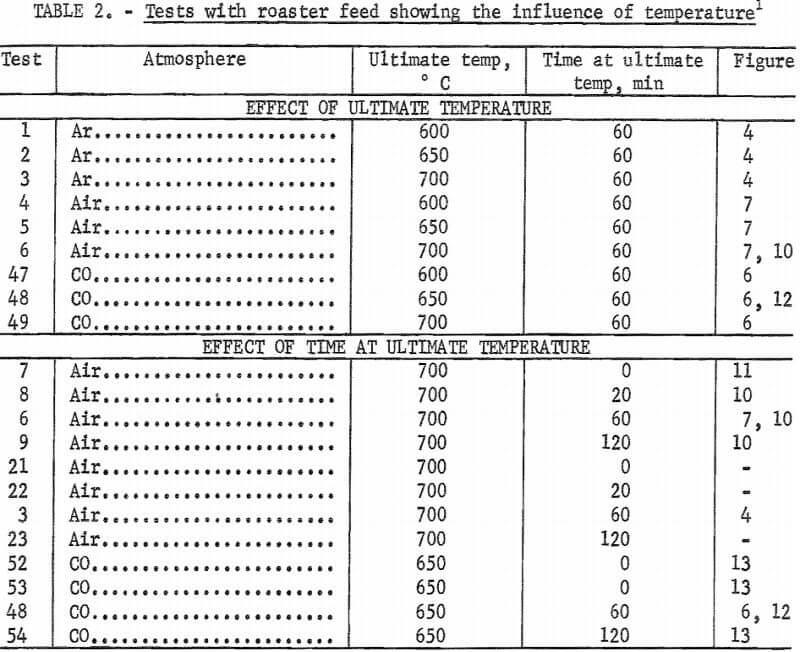
Figures 9 to 11 show the changes in arsenic and sulfur distribution when the bed was heated in air at different rates and held at ultimate temperatures for various times. 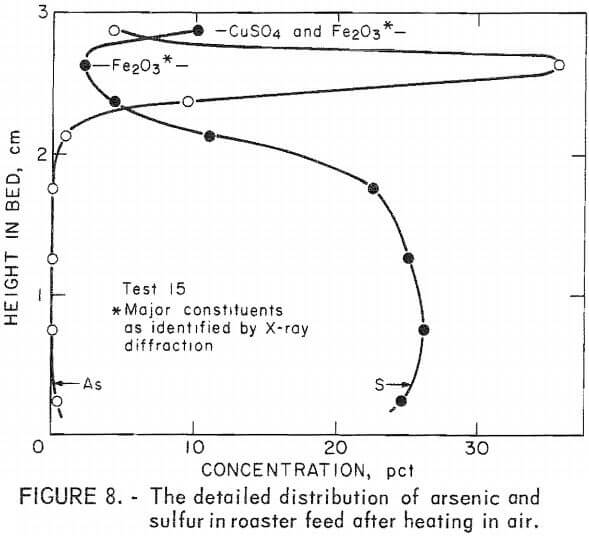 Subtle differences in these curves indicate that the higher heating rates result in more immediate arsenic loss, but once at temperature, time is an important factor in arsenic removal.
Subtle differences in these curves indicate that the higher heating rates result in more immediate arsenic loss, but once at temperature, time is an important factor in arsenic removal.
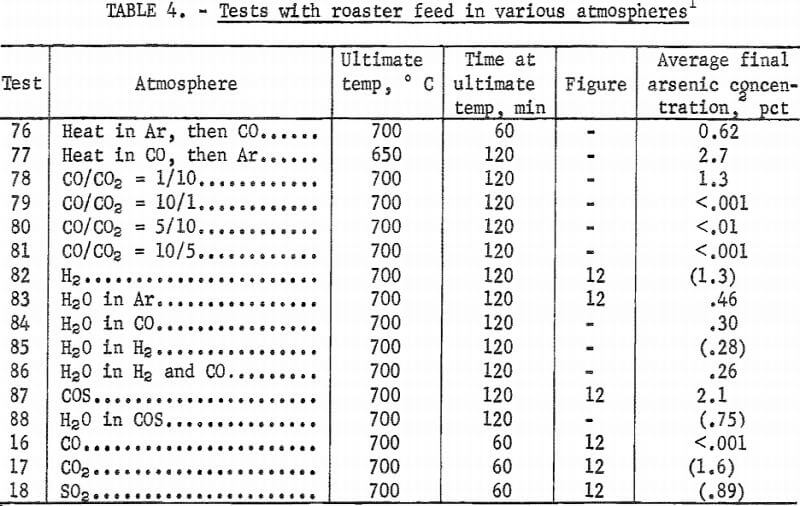
Small amounts of sulfur dioxide, carbon dioxide, and carbonyl sulfide gases were detected by mass spectrometry above the roaster charge heated in carbon monoxide. To determine if these gases were simply reaction products or active reagents for removal of arsenic, each gas was used as the atmosphere in further tests. In addition, hydrogen, because it is a reducing agent, and water vapor (in argon), because it would be a product of hydrocarbon combustion, were used as atmospheres. Results of these tests are shown in figure 12, which indicates that a carbon monoxide atmosphere gives better arsenic removal than any of the other gases tested.
Additional tests were made with carbon monoxide. Heating rate in this gas, studied in tests 48, 50, and 51, only slightly affects arsenic content. Time at ultimate temperature has a more pronounced effect (fig. 13). Tests 76 and 77 confirm that heating in a carbon monoxide atmosphere is not as important for arsenic removal as maintaining roaster charge under carbon monoxide at the ultimate temperature. Final arsenic content in test 76, which used carbon monoxide during the entire test, averaged 0.62 percent while, test 77, which used it only during the heat-up period, averaged 2.7 percent arsenic.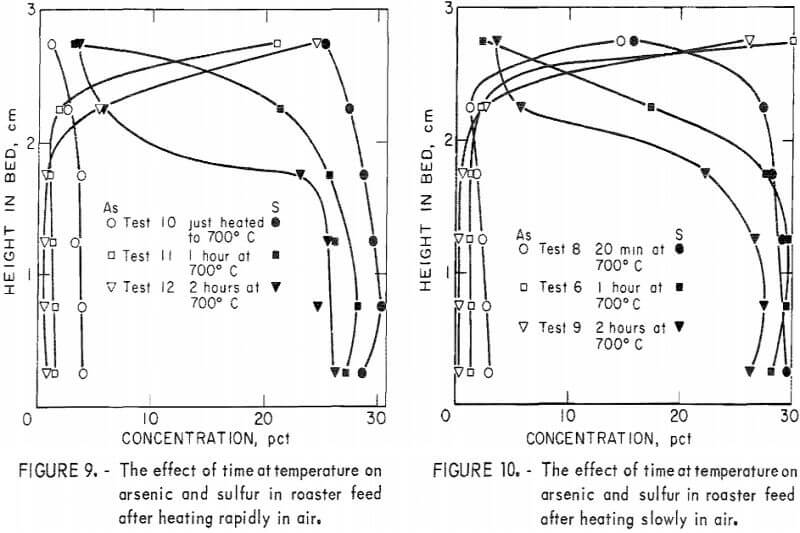
A reducing atmosphere converts arsenic to the volatile element or to volatile arsenous compounds. When arsenic is originally present in the reduced state, a reducing atmosphere maintains this condition. Carbon monoxide appears to be the preferred reductant compared to hydrogen. This could be attributed to the scavenging of sulfur by hydrogen to form hydrogen sulfide. Although carbon monoxide does combine with free sulfur to form carbonyl sulfide, it is not as reactive towards sulfur as is hydrogen. A neutral atmosphere is preferable to an oxidizing atmosphere when removing arsenic since it prevents further oxidation. However, a reducing atmosphere is more efficient because it will reduce oxidized arsenic already present in the feed.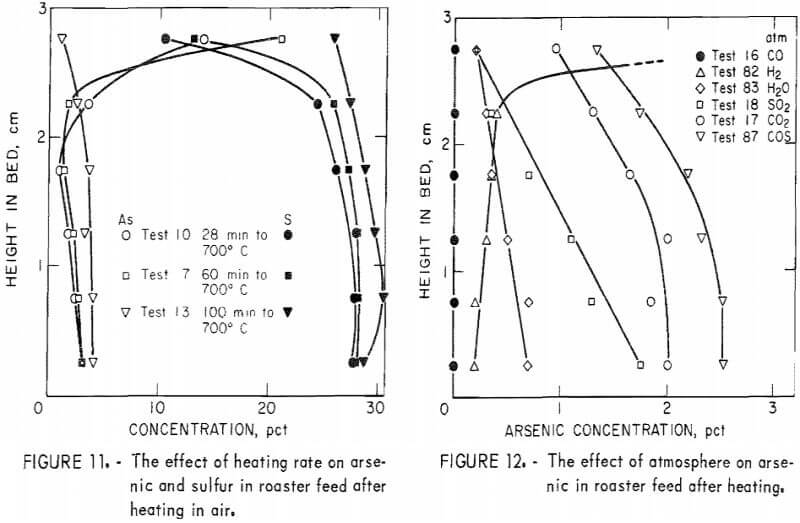
In a static bed under an oxygen-containing atmosphere, a zone exists in which oxidation is taking place. The position of this zone depends upon the diffusion rates of oxygen from above and vapors containing sulfur, arsenic, and other volatiles from the bed below. The volatiles evolve at different rates as evidenced by the residual elemental profiles. When enough sulfur is present, the oxidizing zone may actually be above the bed. As a particular constituent is depleted, oxygen can penetrate further into the bed, and different products thus cause the colored layers observed in the beds heated in air. Thus rapid heating of roaster feed tends to minimize arsenic retention both because sulfur is oxidized instead of arsenic and because the arsenic has a chance to escape contact with arsenate-forming constituents before it is oxidized.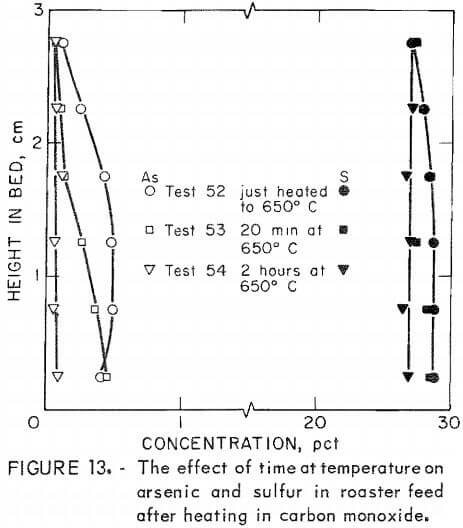
Tests made with roaster feed in other combinations of gases resulted in arsenic concentrations that were essentially uniform throughout the beds. These average arsenic concentrations are shown in the last column of table 4 for the appropriate tests. Test 85 produced a constant arsenic concentration of 0.25 pct except for a single layer of pellets containing 1.9 percent arsenic at the top of the bed. Tests 77, 78, and 88 (table 4) resulted in sloping linear arsenic concentration profiles from bottom to top of 4.10-1.50 pet, 1.80-0.70 pct, and 1.38-0.42 pct, respectively.
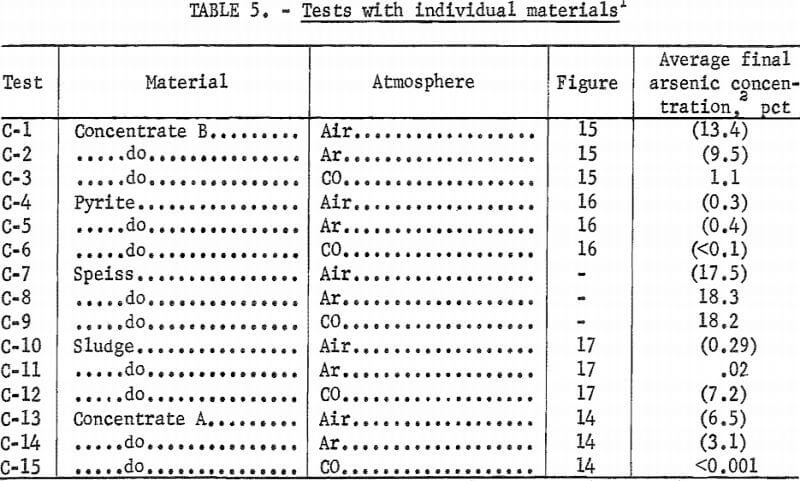
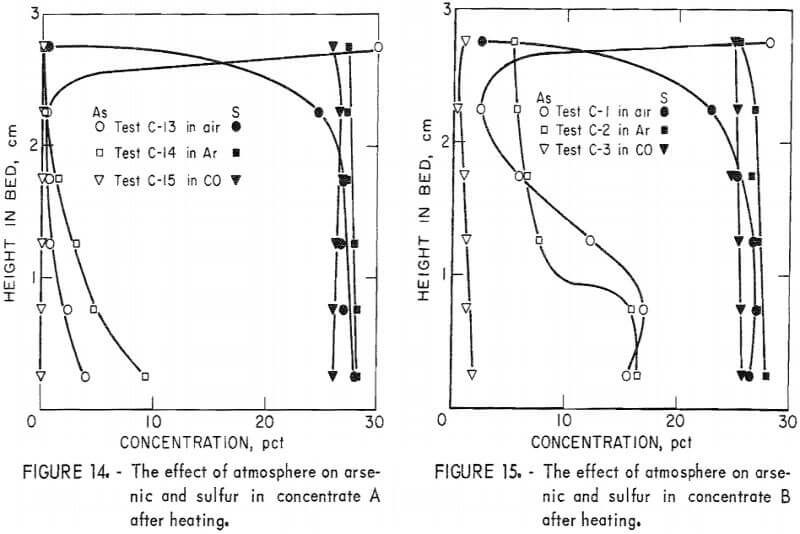
Although the high-arsenic components of the roaster feed were not pure substances in themselves, they represent typical materials which could react in specific ways. With this in mind, beds of roaster charge components were run in air, argon, and carbon monoxide. The test conditions are given in table 5 and the results are shown in figures 14 to 17. Tests were also run with the speiss, but the only change noted was a drop in the arsenic concentration from 18 pct to 13 pct in the top layer of test C-7 (air atmosphere).
Concentrate A is used as a basis for comparing the behavior of arsenic in the other roaster feed components. Arsenic in concentrate B behaved similarly to that in A. The fact that residual arsenic in B was higher than that in A may be attributed to the originally higher arsenic and lower sulfur levels in B. Whether the higher zinc, lead, and antimony in concentrate B had an influence is not known.
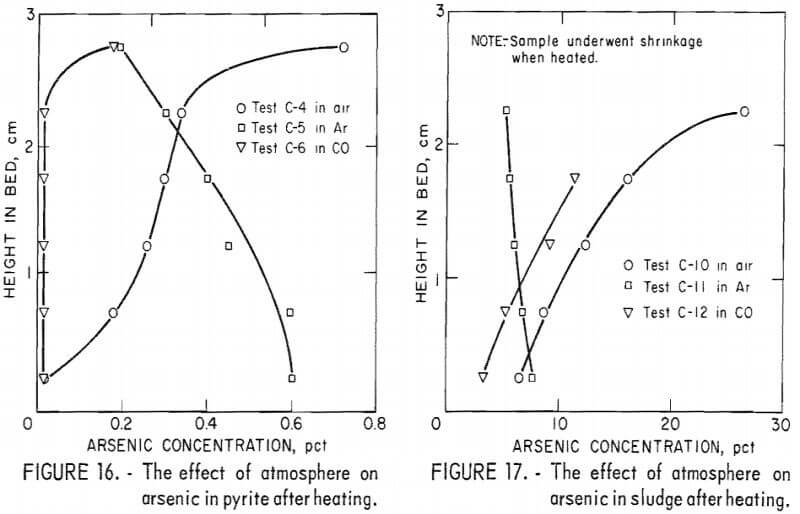
Figure 16 shows that the arsenic concentration in pyrite was considerably reduced in all three atmospheres. The comparatively good removal of arsenic with air can be attributed to the sulfur evolved from the pyrite upon heating. This sulfur vapor reacting with oxygen at the top of the bed kept the oxygen from diffusing into the bed, reacting with the iron and arsenic, and forming nonvolatile arsenic-iron oxides. The results with argon are not completely understood but they indicate diffusion-controlled arsenic evolution consistent with the results of other non-reacting gases above roaster feed (fig. 12).
Sludge, low in sulfur and high in copper, lost only part of the original arsenic (fig. 17). Test C-10 with air shows a migration of arsenic from deep in the bed to the surface where oxygen was available to form arsenates. In carbon monoxide, arsenides may have formed as the sample heated and any oxides were reduced. Such arsenide formation would account for the retention of arsenic under carbon monoxide, especially at the top of the bed. In non-reacting argon, arsenides and arsenates would form only from those compounds already existing in the solid phase. Because there was no increase in arsenic in the upper part of the bed as compared to the lower bed in argon, reaction between vapors of arsenious oxide and elemental arsenic with copper and its compounds may be discounted.
Speiss, essentially nonvolatile arsenides, showed little arsenic loss in these tests. The slight loss of arsenic in an oxidizing atmosphere would be expected as the arsenides pass through the arsenious oxide stage in the process of being converted to arsenates. Oxidation to break down the arsenides, with subsequent heating in a high sulfur and carbon monoxide atmosphere, was subsequently tried as a means of removing arsenic from speiss.
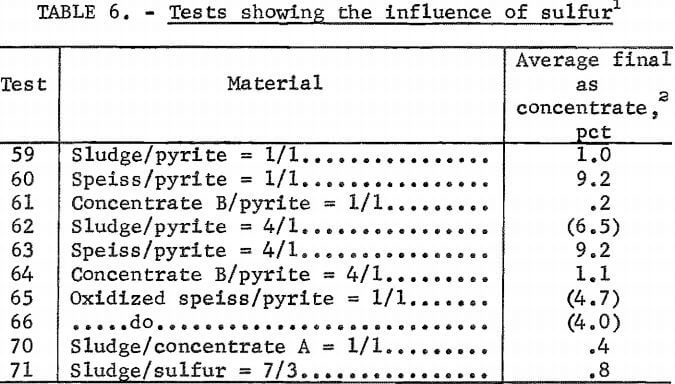
In addition to carbon monoxide, the presence of sulfur appears to be important to arsenic removal, which may be provided by pyrites, other sulfides, or elemental sulfur. Therefore, pyrite, concentrate A, or sulfur was added to those materials which by themselves did not show a ready loss of arsenic when heated in carbon monoxide; then, tests 59 through 75 were run. Table 6 lists the average final arsenic concentrations along with test conditions. Resulting arsenic profiles were essentially constant except for tests 62, 65,
and 66 where arsenic concentration increased about fivefold from bottom to top. Results from the tests listed in table 5 and shown in figures 14 and 17 indicate that increased available sulfur enhances arsenic removal. A notable exception is speiss. Tests C-7, C-8, and C-9 showed no arsenic removal with the exception of a one-third reduction in the top layer of test C-7. Based on this observation, tests 65 and 66 were run with speiss previously oxidized in air and indeed show an improved arsenic removal when compared to test 60.
What happens to the Arsenic in a Static Bed
Static beds of pelletized copper-smelter roaster feed, high in arsenic, were heated in tests using various conditions of time, temperature, and atmosphere. Products of these tests showed variations along the depth of the beds. Using these results, available thermodynamic data, and mass spectrometry of evolving vapors, the following conclusions concerning the removal of arsenic were made:
A temperature of 650° C or higher is required for arsenic removal, even though the volatile arsenic and its compound have sufficient vapor pressure at lower temperatures.
In an oxidizing atmosphere, the nonvolatile As2O5 appears to form.
A nonreacting or neutral atmosphere considerably improves arsenic removal compared to an oxidizing atmosphere.
A reducing atmosphere produces the greatest arsenic removal. In this atmosphere, the lower valence arsenic is vaporized and, in addition, the higher valence arsenic is reduced to a more volatile form.
With sufficient sulfur available, the formation of intermetallies is prevented even in a reducing atmosphere.
Arsenic can be removed to some extent from speiss and other refractory materials by oxidation followed by heating in a reducing atmosphere with a source of sulfur.
