The Bureau of Mines is conducting a program to evaluate technology for the recovery of alumina from domestic resources. Bauxite, the major alumina ore, is mainly imported. In recent years, bauxite-producing countries have formed an organization that attempts to control the supply and price of bauxite. The United States has only limited reserves of bauxite but has large reserves of kaolin, a clay material containing about 39 wt-pct alumina. The development of a process for the recovery of alumina from domestic resources is necessary to insure a continuing supply of alumina to meet the national needs.
The Bureau of Mines in cooperation with several aluminum producers has evaluated several processes for recovering alumina from domestic resources. The major processes studied were hydrochloric acid, nitric acid and sulfurous acid leaching of kaolin, and the lime-sinter process to treat anorthosite.
The objective of this investigation was to verify and expand the experimental data on the sulfurous acid leaching process and to determine if additional large-scale investigations in the Bureau’s miniplant program were warranted.
The sulfurous acid process dates back to the late 1800’s. The process development is attributed to Th. Goldschmidt A.G. which obtained numerous patents. These patents described the different operations of the process pretreatment and leaching of clay, precipitation of monobasic aluminum sulfite, and decomposition of the monobasic sulfite. In 1938, VAWAG at Lippework, Germany, tried the Goldschmidt process on a commercial scale. The alumina product recovered contained excessive quantities of impurities, such as oxides of iron, silicon, chromium, titanium, and phosphorus. A purification step similar to the Bayer process was used to produce a suitable Hall cell feed from the crude alumina. The process was described by Ginsberg and Wrigge. The alumina recovery was reported to be almost 80 pct. In 1962, Peters, Johnson, and Kirby made a cost evaluation of the process.
Interest in the sulfurous acid leaching process is due to the low cost of the SO2 reagent, relatively easy decomposition of the monobasic aluminum sulfite, and the feasibility of substantial recovery of SO2 offgas. The present study includes the determination of the quality of the alumina product, raw material requirements, and possible sources of environmental problems that are associated with the process.
Process Chemistry
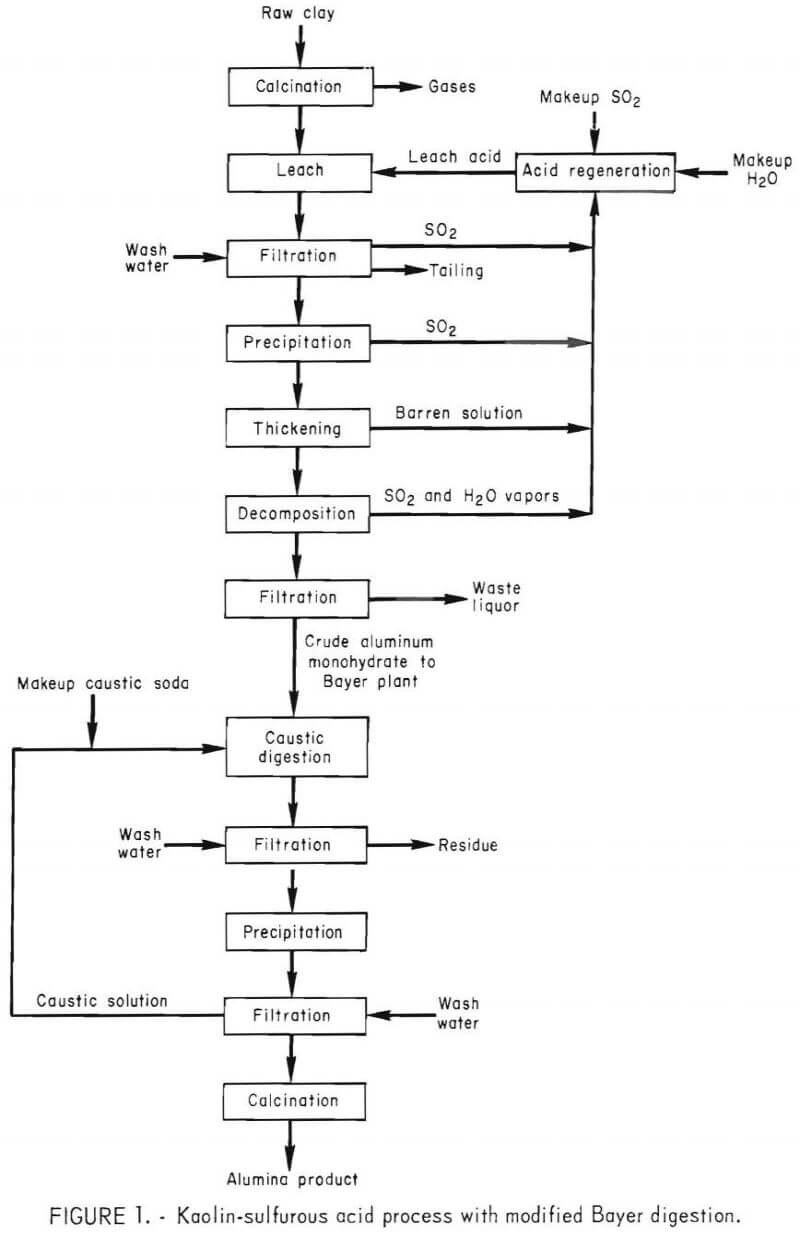
The major steps of the process consist of (1) calcining kaolin at 750° C, (2) leaching the calcine in sulfurous acid (H2SO3), (3) separating the insoluble silica from the pregnant solution by filtration, (4) precipitating mono-basic aluminum sulfite from the pregnant solution, (5) decomposing the precipitate to yield crude alumina, and (6) purifying the crude alumina by caustic digestion and reprecipitation. A flow diagram for the process sequence is shown in figure 1.
The major chemical reaction occurring in the calcining step is the dehydration of kaolin to render the alumina acid soluble,
Al2O3 · 2SiO2·2H2O → Al2O3·2SiO2 + 2H2O………………………………………….(1)
Calcined kaolin is leached in sulfurous acid to yield aluminum sulfite and a silica residue,
3H2SO3 + Al2O3·2SiO2 → Al2(SO3)3 + 2SiO2 + 3H2O………………………………………(2)
Iron and some other minor impurities are also dissolved. Monobasic aluminum sulfite (MAS) is precipitated from the pregnant solution,
Al2(SO3)3 + 5H2O → Al2O3 · 2SO2 · 5H2O + SO2………………………………….(3)
The MAS is thermally decomposed to yield a crude alumina,
Al2O3 · 2SO2 · 5H2O → Al2O3 · xH2O + 2SO2 + (5 – x)H2O…………………………(4)
The crude alumina is digested to yield a sodium aluminate solution,
Al2O3 · xH2O + 2NaOH → 2NaAlO2 + (1 +x)H2O……………………………………..(5)
Iron impurities in the crude alumina remain in the residue. Alumina trihydrate is precipitated from the sodium aluminate solution,
2NaAlO2 + 4H2O → Al2O3 · 3H2O + 2NaOH…………………………………………(6)
After washing, the trihydrate is calcined to yield anhydrous alumina for electrolytic cell feed,
Al2O3 · 3H2O → Al2O3 + 3H2O………………………………………….(7)
Materials, Equipment, and Procedure
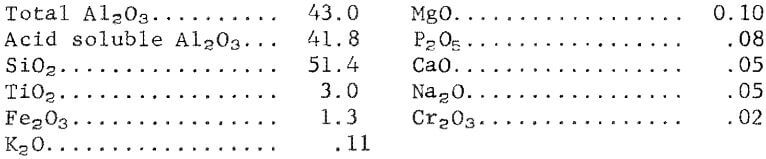
Georgia kaolin was used as feed material. After calcination at 750° C, the kaolin contained the following constituents, in weight-percent:
 The particle size distribution of the calcined material was as follows:
The particle size distribution of the calcined material was as follows:
plus 10 mesh, 4.4 pct; 10 by 100 mesh, 82. 7 pct; and minus 100 mesh, 12.9 pct. Anhydrous SO2 (<0.02 pct impurities) was used.
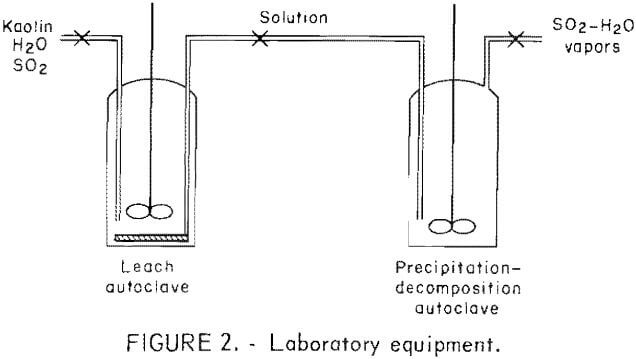
The equipment used consisted of two 2-liter laboratory autoclaves. One vessel was used for leaching and
the other for precipitation and decomposition as shown in figure 2. The leach autoclave was equipped with a coiled, porous metal tube filter that permitted liquids to be withdrawn without opening the reactor. All equipment was fabricated from 316 stainless steel. Air was excluded from the system to minimize the oxidation of soluble sulfites to sulfates.
One-hundred and fifty grams of calcined kaolin and the desired amount of water were put into the autoclave. After closing the autoclave, a predetermined quantity of liquid SO2 was added to obtain the desired sulfurous acid strength. The preparation of H2SO3 solution was expedited by the use of liquid SO2 rather than gaseous SO2. The kaolin was pressure-leached. Pressure within the autoclave forced the pregnant solution through the porous tubing into a second autoclave; thus separating the pregnant sulfite solution from the residue. The leached residue was washed with water and the wash solution was then transferred to the second autoclave. The filtered solution, consisting of the pregnant and wash solutions, was heated to the desired temperature to precipitate the MAS. Excess SO2 was bled off to maintain the required pressure. The resultant slurry contained the sulfite precipitate and barren solution. To adjust the precipitate to solution ratio, the excess barren solution was withdrawn. Decomposition was conducted in the same autoclave as precipitation and was achieved by increasing the temperature and controlling the pressure by venting excess gases from the autoclave. After cooling, the reactor was opened and the slurry was filtered to separate the crude product from the waste liquor.
Solids were analyzed by neutron activation and X-ray fluorescence techniques; solutions were analyzed by atomic absorption.
Experimental Results
Leaching
 Leaching of calcined kaolin was investigated to confirm and establish operating conditions and to determine leaching efficiencies. The following operating variables were determined to be the best for leaching: temperature of 60° C, pressure of 140 to 160 psig, leaching time of 17 hours, 30 wt-pct
Leaching of calcined kaolin was investigated to confirm and establish operating conditions and to determine leaching efficiencies. The following operating variables were determined to be the best for leaching: temperature of 60° C, pressure of 140 to 160 psig, leaching time of 17 hours, 30 wt-pct
SO2 solution, and 10:1 solution-to-calcined kaolin weight ratio.
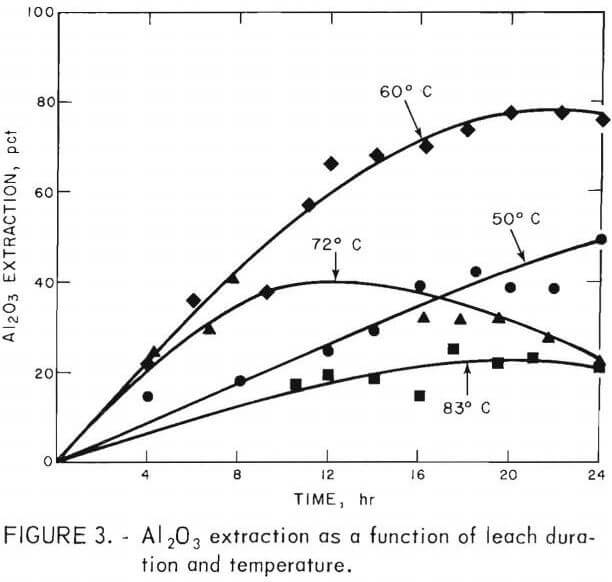
The effects of leach duration and temperature on alumina extraction are shown in figure 3. Alumina extractions presented in this paper are based on total alumina in the kaolin. The best alumina extraction was 80 pct and was obtained after about 20 hours of leaching at 60° C. This produced a liquor containing a maximum of 45 grams of alumina equivalent per liter. If the duration of leaching was extended beyond 20 hours, a decrease in extraction occurred. Leaching at 50° C for 24 hours resulted in an alumina extraction of about 50 pct. At 72° C, the best extraction was 40 pct in 8 hours; beyond 8 hours, the extraction decreased. At 83° C, the maximum extraction was only 22 pct after 17 hours. The decreased extraction at 50°, 72°, and 83° C probably was due to the precipitation of MAS.
Best alumina extraction was obtained by leaching with 30 wt-pct SO2 solutions. Decreasing or increasing SO2 content in the solution resulted in decreased alumina extraction as shown below:
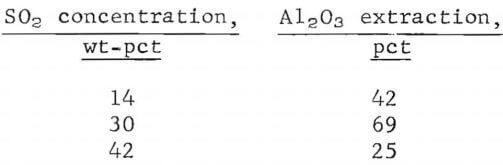
Alumina extraction decreased to 25 pct when solutions containing 42 wt-pct SO2 were used.
Although minus 10-mesh kaolin feed was used, tests showed that kaolin particle size had no serious effect on alumina extraction. Approximately 75 pct extractions were obtained with charges of 88 pct plus 100-mesh kaolin and 80 pct minus 100-mesh pulverized kaolin. Also, an extraction of 71 pct was obtained by leaching minus ½- by ¼-inch pellets that were prepared from the minus 10-mesh kaolin.
Washing the silica residue with water recovered an additional 4 to 10 pct of the alumina entrained as sulfites in the silica residue. A second wash recovered another 2 pct. However, a sulfurous acid wash did not increase the alumina recovery.
Releaching experiments were conducted in an attempt to improve alumina extraction. Leach residue, containing 16.4 wt-pct Al2O3 and 64.0 wt-pct SiO2, was releached for 17 hours with a 30 wt-pct SO2 solution at 160 psig and 58° C. Fifty-nine percent of the alumina was extracted from the residue. This represents an additional 19 pct extraction of the total alumina in the kaolin, or 87 pct overall. The low recovery for the time required does not justify releaching the residue. The residue from the second leach contained 9.3 wt-pct Al2O3 and 70.6 wt-pct SiO2.
Solid-Liquid Separation
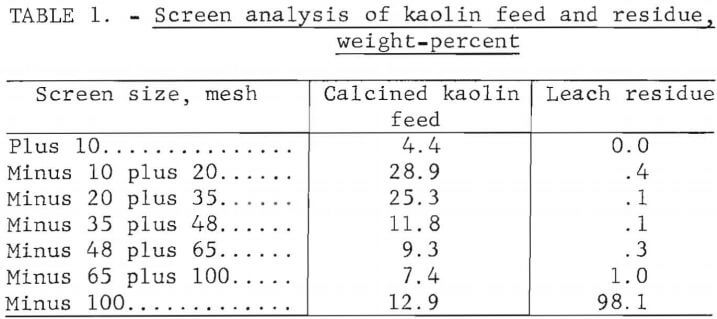
Filtration of the leach slurry was difficult because of the fine particle size of the silica residue. Table 1 shows a screen analysis of the kaolin feed material and the leach residue. Leaching greatly decreased the particle size. Ninety-eight percent of the leach residue was minus 100 mesh. Use of pellets for leaching could minimize the filtration problems caused by the finely divided residue.
Problems associated with filtration of the leach slurry were caused not only by the particle size of the silica residue but also by the precipitation of aluminum sulfites. Electron microprobe analysis of the residue indicated clusters of particles smaller than 1 µm that contained silicon and aluminum, and other distinct crystalline particles that contained aluminum and sulfur, but no silicon. X-ray diffraction analysis of the residue indicated the precipitate was MAS. The precipitation of MAS was caused by pressure fluctuations that occurred during filtration.
The filtration rate of the hot leach slurry was 15.4 ml/cm² hr (3.8 gal/ ft² hr) at a pressure differential of 100 psi. The residue contained 49 pct moisture after filtration. Filter aids and flocculants, such as diatomaceous earth, silica sand, Nalco 2330, Separan MG 200, and Guar Gum C-13, were not effective in facilitating the filtration. Settling rates were measured under an atmospheric pressure of SO2, and the ratio of the clear solution volume to the settled slurry volume (termed solution-to-solid ratio in this report) was determined. The slurry was thickened to a solution-to-solid ratio of 2:1 from an initial ratio of 7.8:1 at a settling rate of 2.6 cm/hr. With the addition of 300 ppm of flocculant Nalco 2330, the slurry settled to a ratio of 1.8:1 at a settling rate of 1,520 cm/hr.
Precipitation
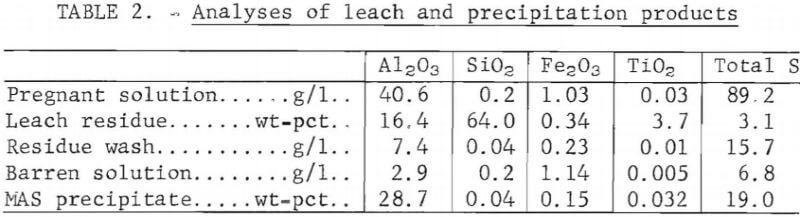
Operating conditions established for precipitation of MAS from the pregnant solution were 2 hours at 110° C and 60 psig. These are essentially the same conditions reported in the literature. About 94 pct of the aluminum in solution was recovered in the MAS precipitate. The pregnant solution contained 40.6 grams Al2O3 equivalent per liter and the barren solution, 2.9 g/l. Analyses of the leach and precipitation products are shown in table 2. X-ray diffraction analysis showed that the precipitates were MAS as reported in the literature.
Additional experiments were conducted to determine the settling rate of MAS in the barren solution. At atmospheric pressure the measured rate was 12 cm/hr. The addition of 150 ppm of Nalco 2330 flocculant increased the settling rate to 27 cm/hr and decreased the solution-to-solid wt-ratio of 7-5:1 to 0.7:1, The final solution-to-solid ratio to which the slurry settled was 0.65:1 at a settling rate of 11 cm/hr.
Decomposition
The following three procedures were investigated for the decomposition of MAS:
- decomposing the MAS with all the barren solution present,
- decomposing the MAS precipitate as a thickened slurry, and
- decomposing the MAS precipitate after filtration and repulping in water.
The operating conditions for decomposing the MAS were a temperature of 140° to 160° C, pressure of 38 to 75 psig and time duration of 1 hour. The steam-saturation pressure at the decomposition temperature was used. This decreased the SO2 concentration, thus minimizing the possibility of the reverse reaction between the SO2 and the alumina product.
The decomposition conditions were modified because the procedure outlined in the literature did not yield the desired results. A decrease in autoclave pressure during decomposition was the major modification. Decomposition at lower pressure resulted in crude products containing less sulfur.
X-ray diffraction of the product from all three procedures showed that major quantities of γAlOOH and a noncrystalline phase were present. The aluminum content of the products ranged from 26 to 33 wt-pct, silica from 0.2 to 1.0 wt-pct, and sulfur from 3.3 to 3.8 wt-pct. More than 70 pct of the sulfur was in the sulfate form; the remainder was sulfite. The product obtained from decomposition of the monobasic sulfite in barren solution was appreciably higher in silica. The iron content of all products was less than 0.1 wt-pct. No significant difference in the products obtained by the three procedures was observed. The recommended procedure is the decomposition of MAS precipitate in a thickened slurry that would allow approximately 50 pct of the barren solution to be recycled to the leach solution.
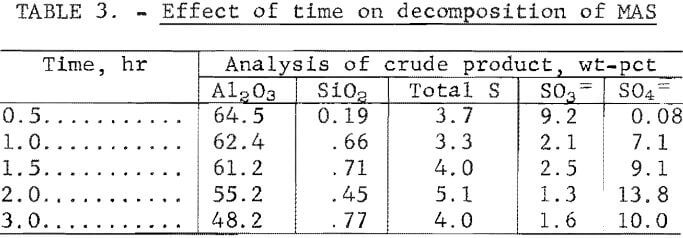
Results from studies to determine the effect of decomposition time are presented in table 3. The results show that decomposition times greater than 1 hour incurred a significant increase in sulfur content in the decomposition products. Other impurities remained at about the same level. A decomposition time of 0.5 hour resulted in incomplete decomposition of the MAS. The Al2O3 content of the product decreased with time, and the water of hydration increased. The decomposition product was filtered from the waste liquor at a rate of 69 ml/cm² hr (17 gal/ft² hr).
Closed Circuit Operation
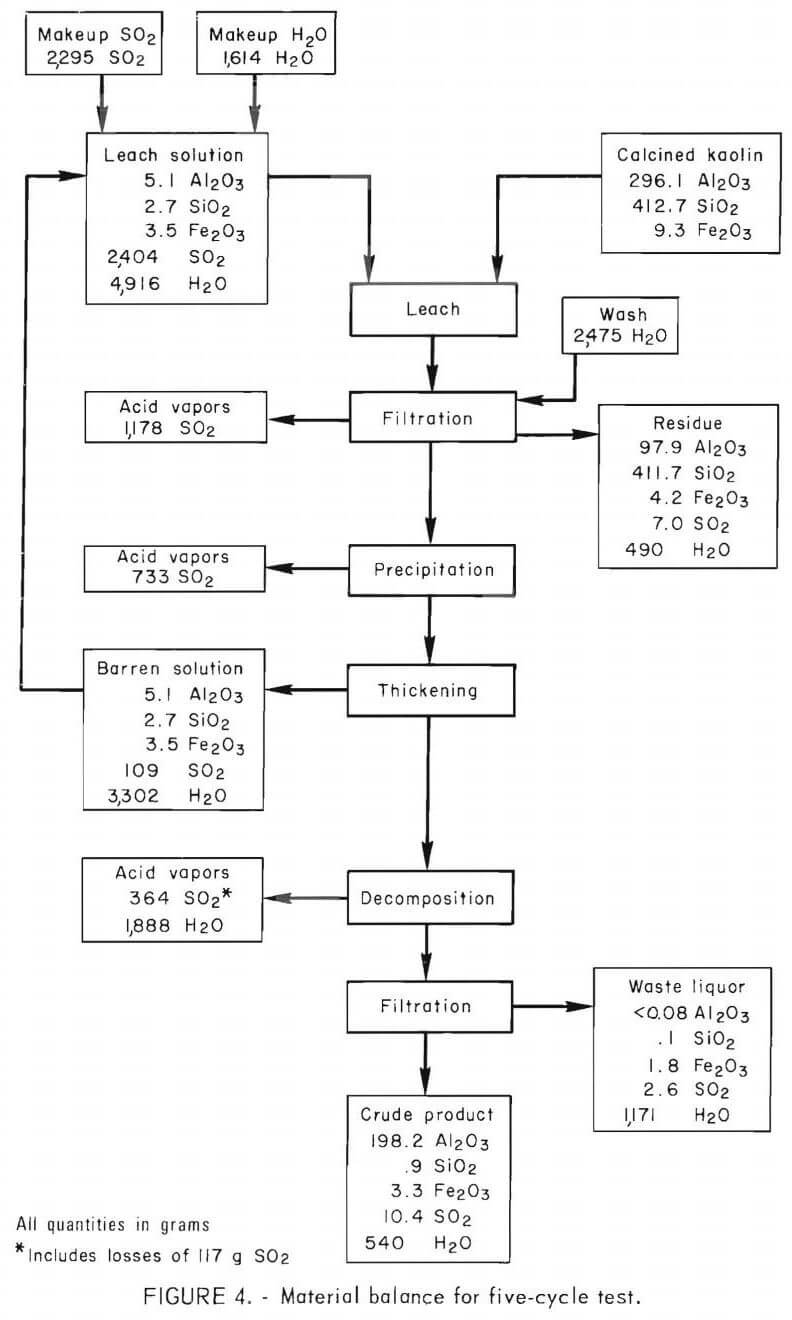
A bench-scale, closed-circuit operation was conducted for five cycles of leaching, precipitation, and decomposition to determine the feasibility of recycling solution, the effect of impurity buildup on the products, and the distribution of major constituents.
The operating procedure was as follows: One hundred and fifty grams of calcined kaolin and 1,500 grams of acid, composed of barren solution recycled from the previous run and additions of makeup SO2 and water, were reacted at 62° C and 170 psig for 18 hours. The leach slurry was filtered in a closed system using a porous 316 stainless steel filter. The silica residue was washed with 500 ml of water, which was added to the pregnant solution. Conditions for precipitation were as follows; temperature, 110° C; pressure, 60 psig; and time, 2 hours. After precipitation was completed, approximately one-half of the barren solution was withdrawn and used as leach solution for the next run. Decomposition of MAS was performed under the following conditions : temperature, 145° C; pressure, 50 psig; and time, 1 hour. Vapors given off during filtration, precipitation, and decomposition were collected in a Dry Ice-cooled autoclave and analyzed by gas chromatography. No attempt was made to recycle the gases.
Analyses of the feed and intermediate products obtained in the five-cycle experiment are given in table 4. Distribution of the major constituents is shown in table 5. Alumina extraction averaged 65.8 pct. Alumina concentration decreased from 37.1 g/l in the pregnant solution to 1.6 g/l in the barren solution. The alumina concentration was <0.07 g/l in the waste liquor. Sulfur dioxide concentration in the leach solution was 321 g/l, of which 49.0 pct was bled off during filtration, an additional 30.5 pct was evolved during precipitation and finally, 10.3 pct was given off during decomposition. Concentration of SO2 in the barren solution was 34.7 g/l and 2.3 g/l in the waste liquor. About 0. 1 pct of the SO2 was discharged in the waste liquor.
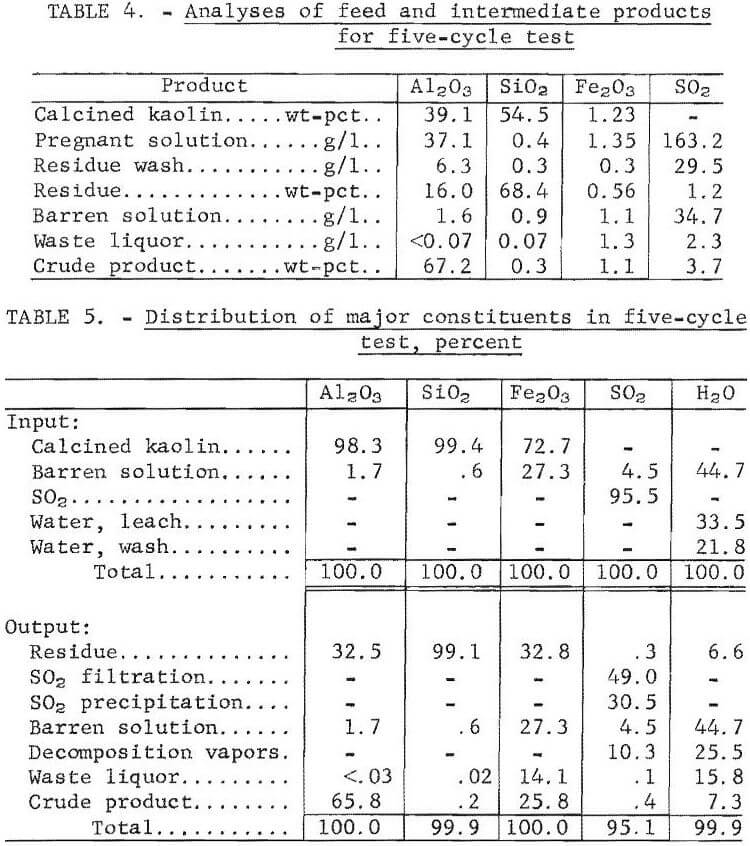
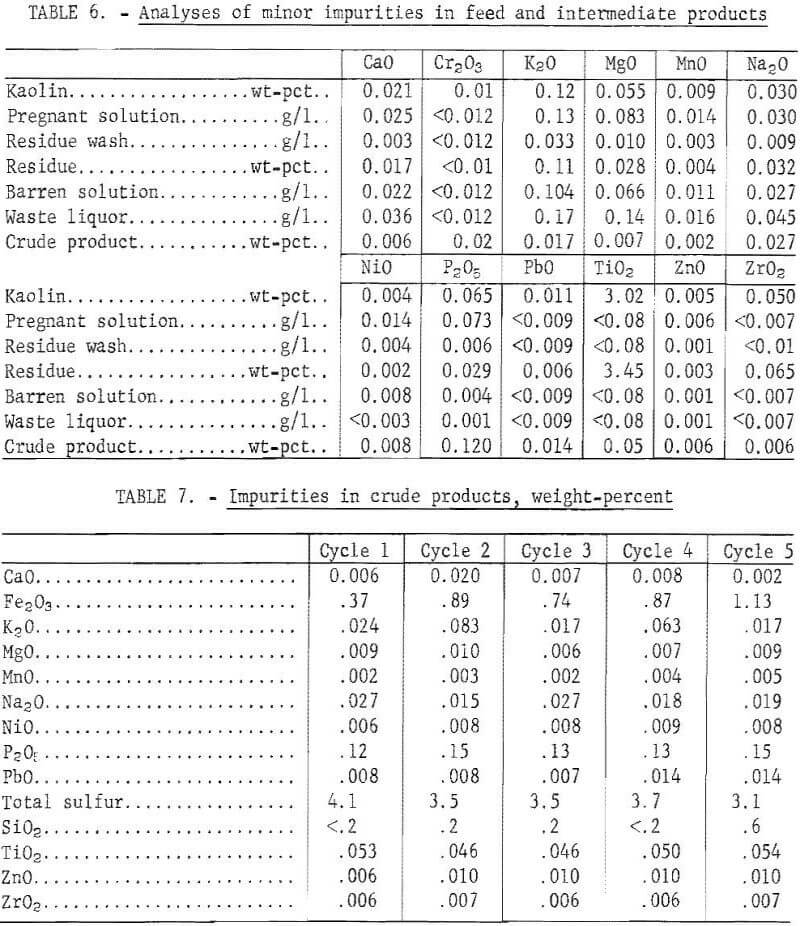
During leaching, 67.2 pct of the iron was dissolved, and 27.3 pct remained in the barren solution after precipitation. The crude product contained 1.1 wt-pct iron, which represented 25.8 pct of the iron in the kaolin, and 0.2 wt-pct silica. The crude product contained 71.8 wt-pct water after filtration and 11 wt-pct after drying. A material balance based on five cycles of integrated operation is shown in figure 4. Impurities present in the feed material and the intermediate products are shown in table 6. Impurity contents in the crude products from five cycles of operation are shown in table 7. Iron oxide was the only impurity progressively increasing during the five cycles. The total sulfur content ranged from 3.1 to 4.1 wt-pct, which included 1.6 to 2.7 wt-pct sulfur as sulfate, and 1.1 to 2.1 wt-pct sulfur as sulfite. No elemental sulfur was present. The contents of the other impurities remained approximately constant.
Sulfur dioxide consumption per pound of alumina recovered was 0.7 pound. Less than 6 pct of the total SO2 used in the process was consumed or lost, while about 94 pct was available for recycle. About 0.8 pct of the SO2 was lost in the residue, waste liquor, and crude product. About 70 pct of the sulfur contamination in the crude product was present as sulfate; the remainder was sulfites. Sulfur is multivalent and easily oxidized from sulfite to sulfate in solution. Sulfur dioxide was not reduced to elemental sulfur. Sulfur dioxide losses caused by leakage from the equipment amounted to 4.9 pct. Escape of SO2 and disposal of liquid and solid wastes may present difficult environmental problems.
Analysis of the offgases collected during filtration and precipitation showed SO2 and no water vapor. The detection limit for water was less than 0.1 pct. Gases given off during decomposition contained from 6 to 16 wt-pct SO2 and 84 to 94 wt-pct water.
Purification of Crude Product
A modified Bayer digestion was necessary to remove excessive quantities of impurities, such as silica and iron, from the crude product from the decomposition of MAS. The purification was required to produce an acceptable cell feed for electrolytic reduction. Because the Bayer process is well known, this phase of the process received only a cursory investigation.
Only 75 pct of the alumina was extracted using an atmospheric leach at 90° C. The decomposition product, identified by X-ray diffraction analysis as aluminum monohydrate, requires higher temperatures and pressures for digestion.
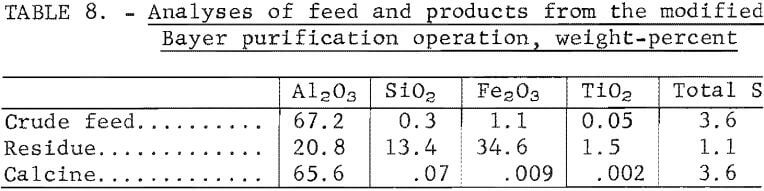
The crude products obtained from the five-cycle operation were combined for use in the purification test. The crude product was dissolved in a caustic solution containing 373 grams of NaOH/l. Digestion required a temperature of 250° C, pressures up to 500 psig, and time of 2 hours. Ninety-eight percent of the alumina contained in the decomposition product was dissolved. After filtration to separate the insoluble residue from the liquor, the liquor was cooled to 50° C and seeded with aluminum trihydrate. A hydrolyzed precipitate was obtained and identified by X-ray diffraction as gibbsite, α-Al(OH)3, and bayerite, β-Al(OH)3. The precipitate was calcined at 1,200° C. The calcined alumina was identified as β-Al2O3 by X-ray diffraction analysis.
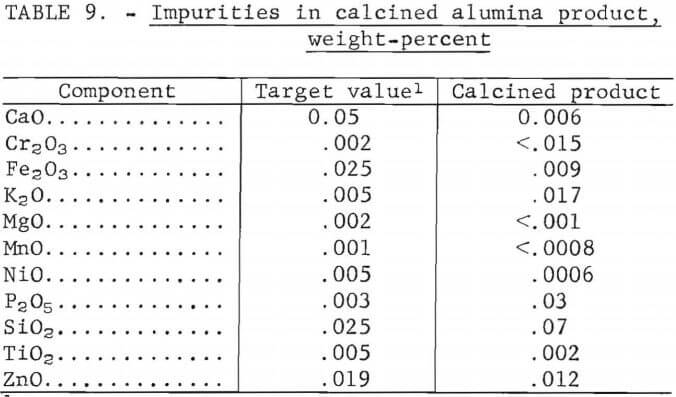
Analyses of the crude feed, the digestion residue, and the calcined product are shown in table 8. Sulfur in the calcined alumina product reached 3.6 wt-pct, which is probably higher than acceptable for electrolytic cell feed. Maximum sulfur specifications are not available from alumina producers. Other impurities are listed in table 9 and are compared with the generally accepted values for cell-grade alumina. The product exceeds the target values for Cr2O3, K2O, P2O5, and SiO2. The results indicate that the alumina obtained was not a suitable feed for reduction cells.
Miscellaneous Observations and Data
Throughout these tests, 316 stainless steel withstood severe conditions of SO2 concentrations up to 30 wt-pct and temperatures up to 150° C. Corrosion did occur on components in contact with solutions containing more than 15 wt-pct SO2 and temperatures in excess of 160° C. In these incidents, X-ray fluorescence scan indicated iron, chromium, and nickel present in the leach solution.
Conclusions
Sulfurous acid leaching of calcined kaolin with a caustic purification does not produce alumina that meets cell feed specifications for alumina purity. A major contaminant is sulfur, which is not decreased by caustic purification. The process requires long leach duration and yields low alumina extraction. Escape of SO2 because of leakages in pressurized equipment presents an environmental problem. Sulfites and sulfurous acid present in the waste liquor require treatment to render the solution innocuous before disposal. For these reasons, the Bureau has concluded that larger scale investigations of the sulfurous acid leaching process, under its cooperative miniplant program, are not warranted.
