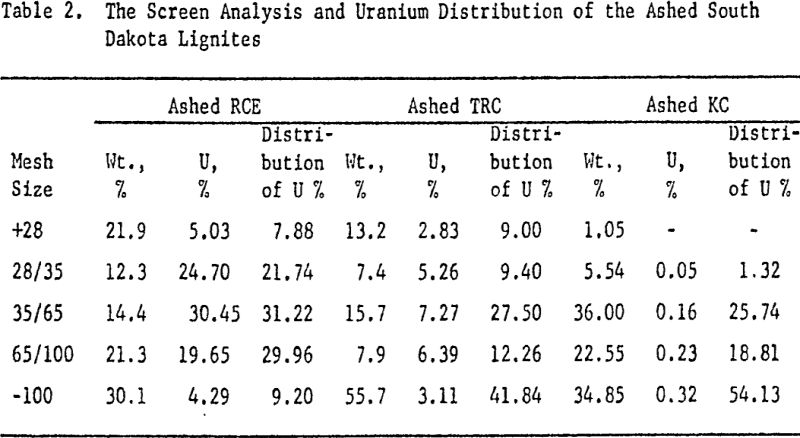
Compared to the uranium ores in Colorado Plateau and New Mexico, the uranium-bearing lignites in North and South Dakota are relatively difficult to process. This is due chiefly to the irregular mineralization of the lignites, and partly to the non-uniform distribution of uranium within the deposits.
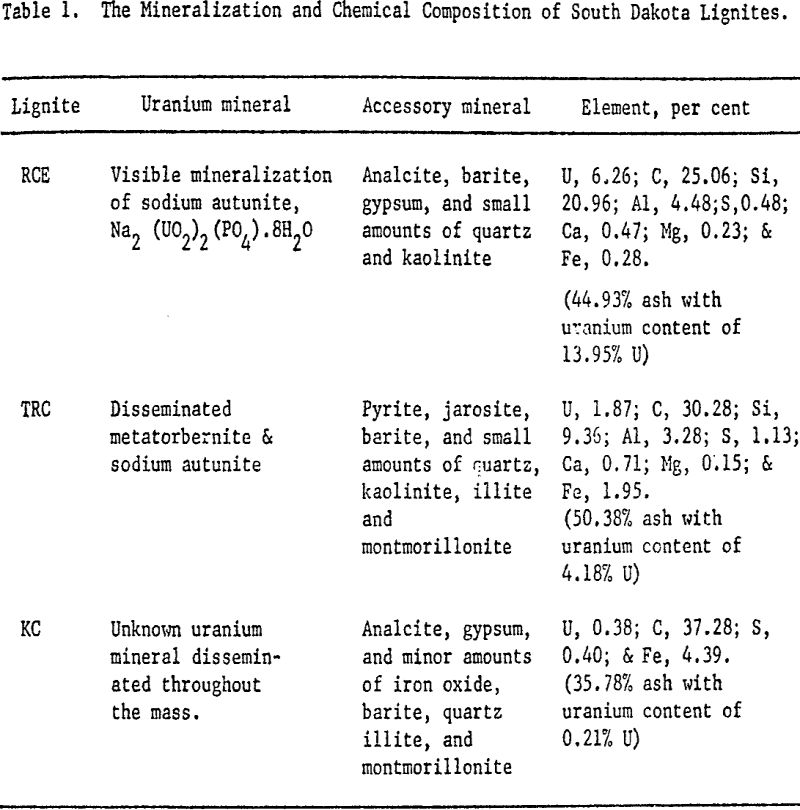
The three lignite samples used in this investigation were collected from Harding County, South Dakota. The uranium minerals require some clarification. Uranium in primary minerals, such as pitchblende, occurs in the reduced or four-valent state combined with oxygen as UO2, or in a partially oxidized state combined with oxygen as U3O8. In secondary uranium minerals, the oxidized or hexivalent uranium ion combines with oxygen to form the uranyl ion, UO2+². It is this latter form that appears in the samples used in this investigation.
Except where otherwise stated, leaching experiments were performed under the following conditions: pulp density, 16.7% solid for RCE and TRC and 10.7% solid for KC; acid concentration, 5% H2SO4; agitation, 24 hours on rolls at a speed of 24 r.p.m.; and temperature, 20°C.
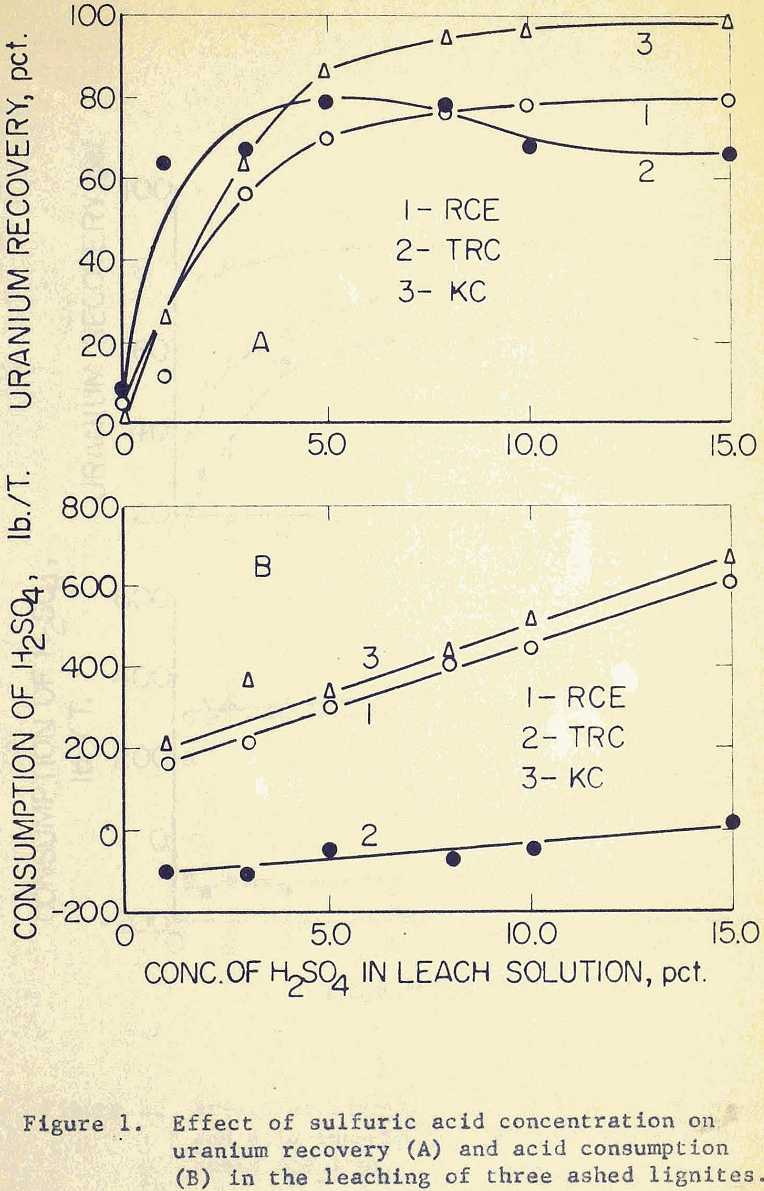
Tests were performed to determine the effect of sulfuric acid concentration on the leaching of ashed lignites. The results, as given in Figure 1A, show that uranium recovery increases with an increase of acid concentration, even though the rate of recovery drops off around the concentration of 5% sulfuric acid. This optimum concentration of about 5% sulfuric acid is in general agreement with accepted mill practice. The lowest uranium content of sample KC means that it would require less oxygen for complete dissolution. It follows, therefore, that an ashed lignite high in uranium content would require more oxidation, and under the employed leaching conditions would give low uranium recoveries. This is exactly the case with sample RCE. Under the same assumption, sample TRC would be expected to react in a manner midway between KC and RCE, since the former has an intermediate uranium content.
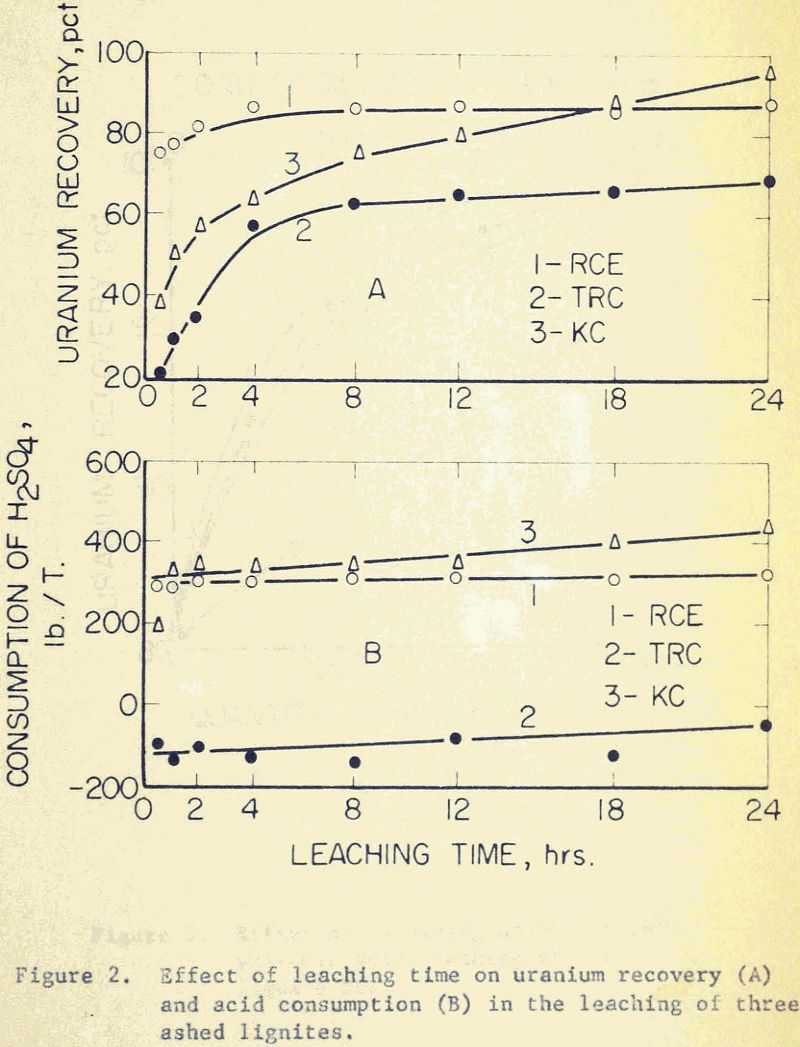
In order to determine the effect of leaching time, the three samples were agitated with 5% H2SO4 over a time range from 15 minutes to 24 hours. The rate of recovery, however, is not the same for all the samples. At short leaching times, the visible uranium mineral sodium autunite in RCE is considerably easier to dissolve than the finely dissemenated ones in KC and TRC.
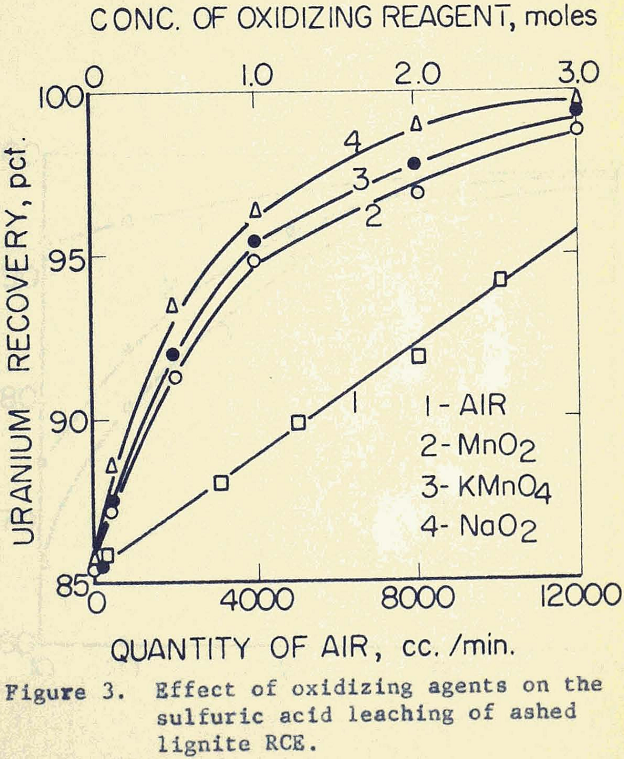
It is significant to note that the addition of air within the practical range does not give sufficient oxidation to recover the uranium as completely as desired. On the other hand, uranium recovery rises to 95% in the presence of 1.0 mole of a chemical agent.