Table of Contents
Acid mine-water drainage is a serious problem with many mines in the Tri-State zinc and lead mining district. Particularly is this true when large volumes must be considered in unwatering old mines that have refilled with water which has dissolved the products of oxidation and has become chalybeate in character.
Original Mining Results in Oxidation
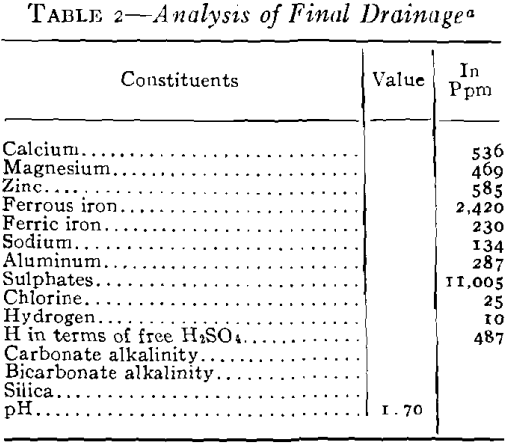
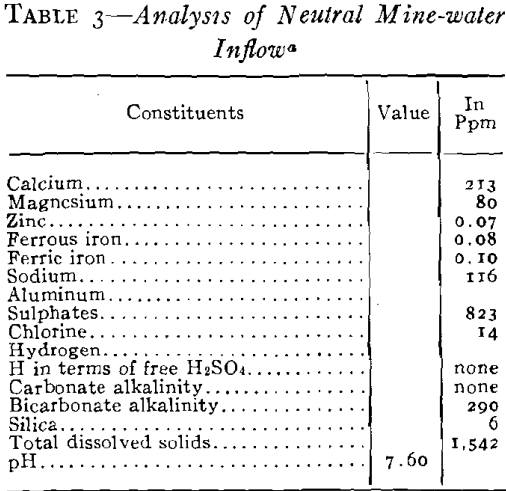
As plans for unwatering progressed, it soon developed that the mine-water pools had become strongly acid in character and carried large amounts of sulphates in solution. This occurred in the following way. When the original sulphide water was removed, shafts sunk, and mining carried on over a number of years, ferrous sulphate was formed by the spontaneous oxidation of the pyrite beds in contact with air admitted through churn-drill holes and mine openings. These products of oxidation can be seen today in the old mines of the district as crusts of crystalline sulphates on the walls of stopes and as stalagtites and stalagmites formed under roof drips. Ferrous sulphate predominated, but large amounts of other sulphates were precipi-
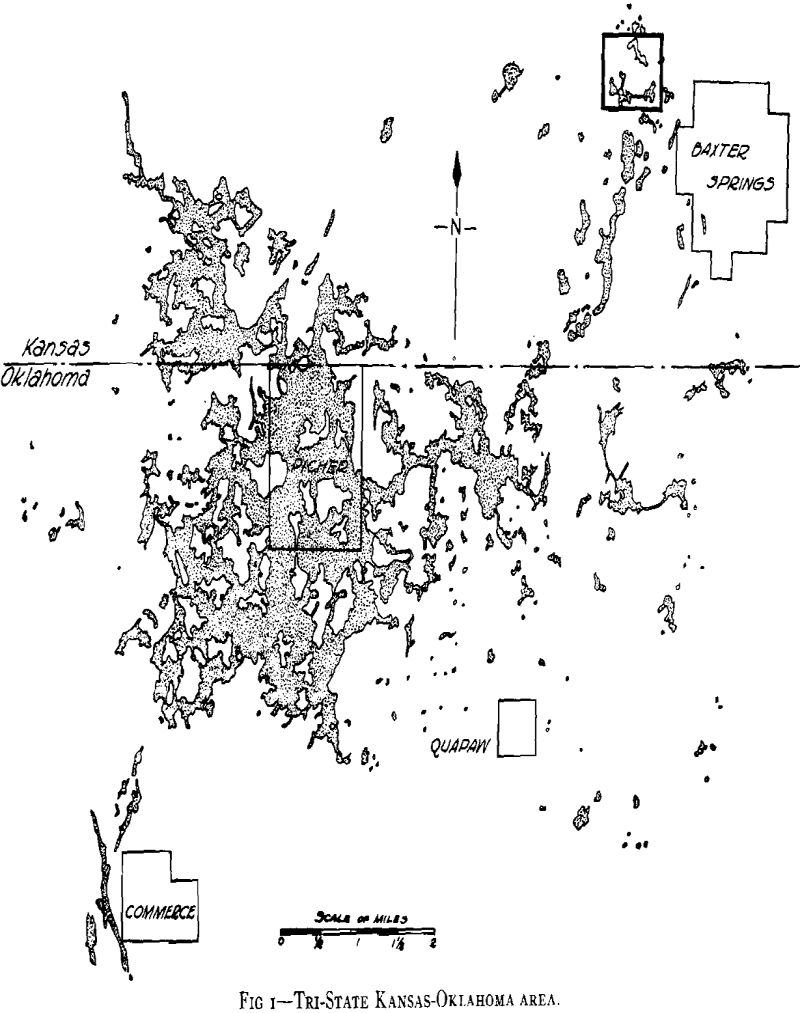
tated as oxidation products of the alkaline carbonates and bicarbonates. The oxidation covered a large area because of the fact that the shear zones were very open and permitted easy circulation of air during the period of mining. With the cessation of mining operations during the depression the entire area refilled with water and the accumulated sulphates formed by this oxidation were dissolved. As the water table rose, a large amount of ferric sulphate was also formed from the oxidation of the ferrous sulphate together with the basic ferric sulphate.
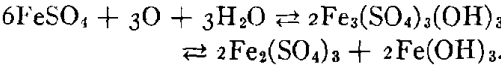
Stream Pollution Problem
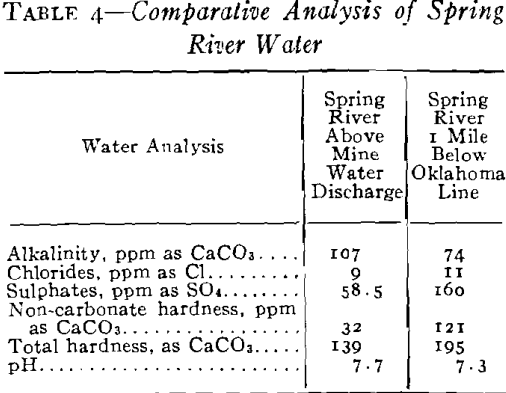
It was necessary to pump a large pool of this type water in order to unwater the mining area, and it was evident that if this water was discharged into stream drainage without treatment, a serious stream pollution would be created. Not only would it have detrimental effects on the use of Spring River from a recreational standpoint but it would also bear on the fishery problems in the Pensacola Dam Impoundment of the Grand River into which drainage would flow via Spring River. The effect of high concentrations of this type of water would be severe on fish life because of the acidity, the toxic metals in solution, the turbidity which would be created by the precipitates and the impoverishment of the dissolved oxygen of the river waters by the oxidation of the ferric iron. Based
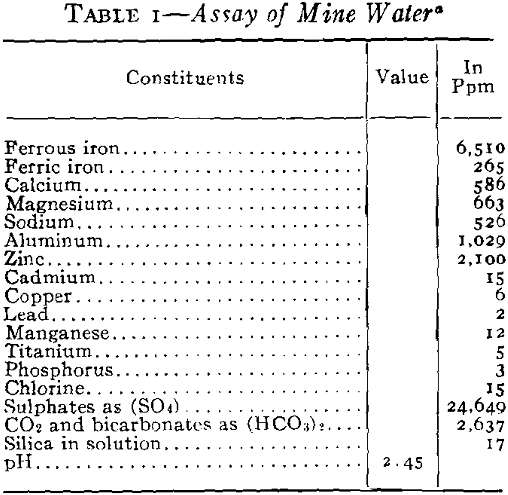
Effects of Discharges into River and Relative Reaction
You will note that discharges into Spring River carried about 500 ppm ferrous iron still in solution. Experiments had shown that at a river stage of 3 ft, the approximate flow of Spring River was 44,000 gpm and that 10 pct or about 4400 gpm of mine water running not more than 500 ppm ferrous and at a pH not lower that 4.5 would permit a dilution not injurious to fish life. The control of dilution to the limits mentioned had no effect upon the iron hydrates still left at this stage, and all the iron remaining was neutralized and precipitated at once by the excess of alkalinity and oxygen in the river and the precipitates settled out within the first mile of flow.