In 1939 one of the authors described advances in carbon-cyanidation for the period 1932 to 1939 and included: (1) the dissolution of gold in an ore pulp by cyanide and its simultaneous adsorption by carbon, (2) the stage addition of adsorptive carbon and its movement countercurrent to the flow of the ore pulp, and (3) the recycling of adsorptive carbon.

During the progress of the work involving the use of coarse adsorptive carbon it was realized by the authors that if such adsorptive material were to be employed on a commercial basis either a lower cost carbon than that available during the testing period must be provided or the problem of desorbing the gold from the carbon and the re-use of the carbon must be solved. Referring to the availability of a lower-cost coarse carbon, although considerable progress has been made in reducing the cost, insofar as the authors are informed, the cost of such carbon is still too high to warrant employing it once and destroying it by burning for the recovery of its adsorbed gold. Recent work has demonstrated that carbon derived from ore pulps can be almost completely desorbed of its gold and silver and the carbon re-used for further adsorption.
Stagnant Pulp Modification: The Eagle-Picher Mining and Smelting Co., as previously mentioned, operated a 25-ton capacity pilot plant to confirm results which had been obtained in the laboratory in eliminating agitation during the dissolution of gold from an ore pulp by cyanide and its adsorption by carbon.
Lime, cyanide, and carbon were added to the charge at the rates of 0.4, 0.5, and 3.6 lb per ton of tailing, respectively. Soft or hard wood charcoal, ground to from 48 to 150 mesh and activated at the plant by heating to 1400 °F and cooling without quenching, was used.
Approximately 4000 tons of this material were treated on an experimental basis. The heads assayed from 0.085 to 0.11 oz gold per ton. The extraction varied from 66 to 79 pct and the carbon flotation concentrate assayed from 16 to 23 oz gold per ton. Barren solutions varied from 0.0042 to 0.0005 oz gold per ton depending upon the fineness of grinding the carbon and its adsorptive efficiency.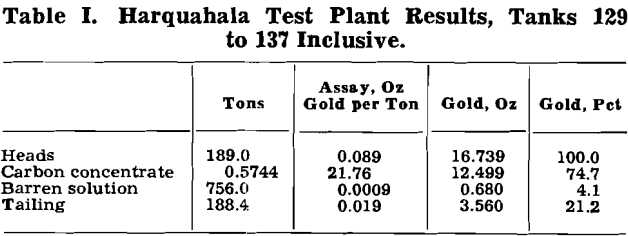
Adsorption With Revolving Screens Containing Carbon:
G. H. Roseveare, of the Arizona Bureau of Mines, working with Chapman in the laboratories of the University of Arizona was the first, to the author’s knowledge, to construct a horizontal, revolving-screen test unit attached to a laboratory mechanical-type agitator. A few months later Chapman and others did the experimental work involving the permissible volume of carbon in a screen and the size of screen required in mill operation. A 5×5 ft Wallace agitator and a 12×15 in. revolving screen were used for this work.
This modification differs from other modifications in that the steps of dissolving the gold, adsorbing the dissolved gold, and separating the carbon from the ore pulp are combined and take place as a continuous process in a single step. It should be noted that the combining of the three steps was described for a stagnant pulp and that the revolving screen only served to make the batch process of the stagnant pulp modification a continuous process. The countercurrent movement of the carbon may be used as in other modifications.
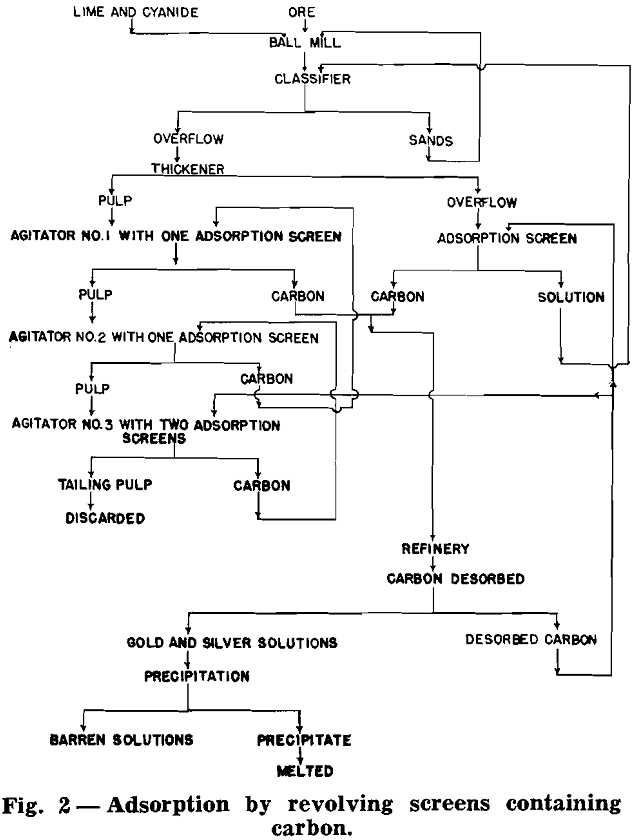
The test was operated as described for 15 hr and for the remaining 48 hr a scavenging screen was added to the tailing launder. The tailing pulp was allowed to flow from the launder to the top of the revolving scavenging screen and a rounded depression in the launder permitted the screen some submergence in the pulp.
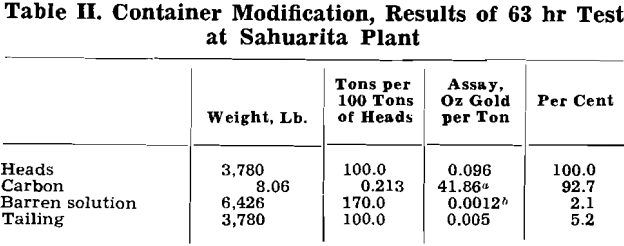
Desorption of Gold and Silver from Carbon: The desorption of gold and silver from a carbon that has been in contact with an ore pulp apparently de¬pends upon the shifting of the equilibrium encountered between adsorption and desorption. Four methods of shifting the equilibrium succeeded in desorbing the gold and silver. The four methods comprised: (1) the use of a solvent in conjunction with a large excess of precipitant in order to remove the gold from the receiving solution as the desorption progressed; (2) the use of a solvent in conjunction with electrolysis to accomplish the same purpose; (3) the use of a solvent and large volumes of solutions, added in stages, to keep the concentrations of the gold and silver in the receiving solu-
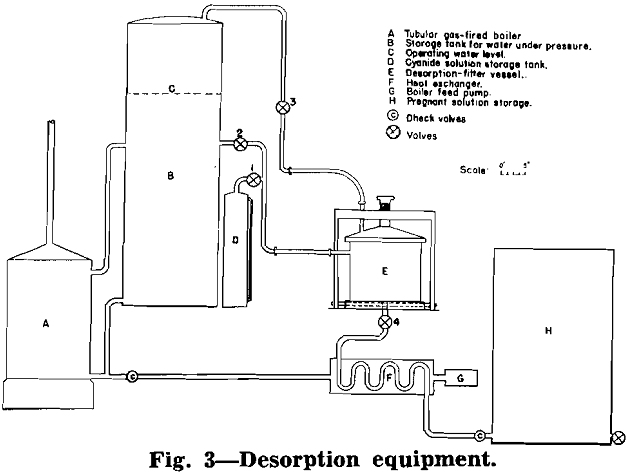
tions sufficiently low to accomplish desorption; and (4) the use of higher temperatures by employing hot solvent solutions in a pressure chamber. The authors believe that the last method given is the most feasible as this method is very rapid, has the lowest reagent cost, and accomplishes the desorption with the minimum volume of desorbing solution.
Cyanide was added for the test described at the rate of 2 lb per ton of carbon per cycle or a total of 10 lb of cyanide per ton of carbon. With other carbons it was necessary to add greater amounts of cyanide. Tests to determine minimum cyanide requirement for a given carbon have not been made to date.
Referring to the barren solutions, two barren solutions are included in the tabulation. The first is the solution discharged from the third agitator, and the second, the solution leaving the scavenger screen of the tailing launder. The figures in the tabulation show an increase in gold content of the barren solution with petroleum coke and shell carbon in the recycling of the desorbed carbon without reactiva¬ion. With petroleum coke, the fresh activated carbon yielded a barren solution of 0.0010 oz gold per ton after the scavenging screen, a barren solution of 0.0018 oz with the recycling of the carbon after one desorption, and a barren solution of 0.0020 oz with the recycling of the carbon after two desorptions.