The adsorption behavior of sodium oleate on brucite, Mg(OH)2, has been studied by Fourier transform infrared spectroscopy. Both internal reflection and diffuse reflection (DRIFT) methods were used to characterize the adsorbed oleate on brucite. DRIFT measurements were shown to be quantitative, and an equilibrium adsorption isotherm was obtained. The conformational behavior of the adsorbed oleate was investigated as a function of adsorption density. It was found that at sub-monolayer coverage the alkyl chains of the adsorbed oleate had more gauche character than at multi-layer coverages.
Experimental Materials and Procedure
All materials used were reagent grade. The Mg(OH)2 powders were from Dead Sea Bromine, Inc. (Israel). The MgO single crystal was also the product of Dead Sea Bromine, Inc. Sodium oleate from MC&B (Norwood, OH) was used without further purification. Sodium hydroxide from Mallinckrodt (Paris, KY) was used for pH adjustments, while the KBr used as the matrix in the FT-IR experiments was from Aldrich (Milwaukee, WI).
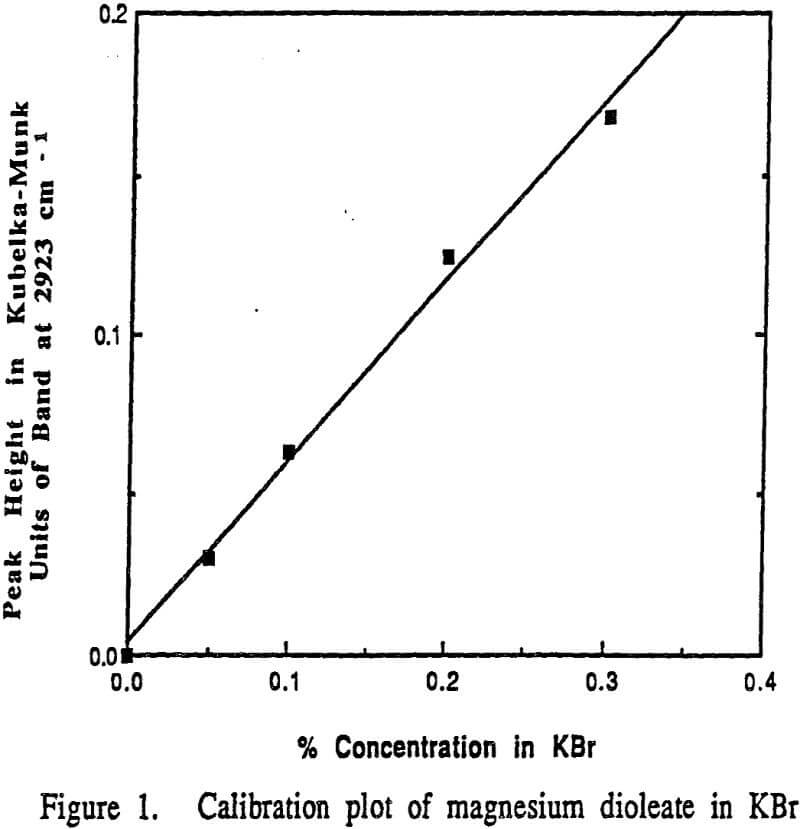
To quantitatively assess the adsorption density of oleate at the brucite surface, the following procedure was followed. Magnesium dioleate was precipitated from solution to provide a suitable calibration material. The magnesium dioleate utilized in the calibration calculations was prepared by adding 3.04 grams of sodium oleate to 0.1 liters of water which had been adjusted to pH 10 by the addition of 0.1 M NaOH. to give a 0.1 M solution of sodium oleate. A solution of 0.1 M magnesium chloride was prepared by the addition of 2.03 grams of MgCl2 to 0.1 liters of water followed by pH adjustment to pH 10 by the addition of 0.1 M NaOH.
The adsorption of oleate by magnesium hydroxide was accomplished by adjusting 0.4 liters of water to pH 9.25 with NaOH. Sodium oleate was added to this alkaline solution to obtain the desired surfactant concentration. One gram of Mg(OH)2 was obtained by weighing 2.77 grams of magnesium hydroxide slurry, which contained 36.4 wt.% solids. The sodium oleate solution was then poured into the weighed slurry and mixed for 1 hour at room temperature. The magnesium hydroxide particles (containing adsorbed oleate) were then filtered to remove the water and dried at 100°C for 1 hour.
The FT-IR spectra of the surfactant treated Mg(OH)2 were obtained in a manner similar to the calibration samples. In this case an untreated Mg(OH)2 spectrum was obtained to use as a reference and both the treated and untreated Mg(OH)2 samples were mixed to 3 wt.% prior to 2 minutes mixing in the Wig-L-Bug mixer. The DRIFT spectra were recorded by ratioing against a KBr single-beam background spectrum.
Results and Discussion
It can be seen from this plot that the DRIFT technique under these conditions can be used quantitatively. The curve shown in Figure 1 was used to determine the adsorption density of oleate on Mg(OH)2 at pH 9.25 and 25°C.
From a scanning electron micrograph of Mg(OH)2, It can be seen from this micrograph that the Mg(OH)2 particles have a very distinct platelet shape. The particles are approximately 1 micron in diameter and are approximately 0.1 micron thick. Also, the particles are not aggregated so that they can be used without further treatment. These powders were used as substrates to determine the adsorption density of oleate on brucite.
Recently, it has been shown that IR spectral data can be used to determine the conformation of adsorbed species in flotation systems. In these systems the conformation state refers to the relative energetic state of -CH2 groups along the alkyl chain of the adsorbed molecules. In general terms, the alkyl chain can be divided into groups containing four consecutive methylene groups. In these groups rotations about the central carbon-carbon bond will occur. These rotations are described as either a trans conformation or a gauche conformation, because these are the two stable energetic states.
For both flotation and composite adhesion studies, the hydrophobicity after surfactant adsorption is important. The most common means of assessing hydrophobicity is through contact angle measurements. For these measurements a flat substrate of size great enough to accommodate viewing of a liquid or gas drop (>1mm) is necessary.