Using a Mark IV surface force apparatus, adsorption of dodecylamine hydrochloride on mica has been studied at pH 5.7 in the presence and absence of octanol. With amine alone, the repulsive forces between mica surfaces are reduced due to charge neutralization and attractive hydrophobic force; however, the latter is of relatively short-range with its decay length less than 2 nm.
F/R = -Cexp (-H/D0)……………………………………………………………………..(1)
in which R is the curvature of the mica surface, D, decay length, and C is a constant. For very hydrophobic surfaces, the hydrophobic force can be best described by a double exponential function.
Surface Forces Measurements
The direct force measurements were made using a Mark IV surface force apparatus from Anutech Pty. Two molecularly smooth mica surfaces were silvered on one side and then glued silver-side down onto silica disks using Epon 1004 from Shell Chemical Co. The separation distance between the two mica surfaces was measured using multiple beam interferometry based on the method of fringes of equal chromatic order (FECO).
Flotation Experiments
Flotation experiments were conducted using a standard microflotation cell. The silica sample was conditioned initially in a known RNH3Cl solution for 30 minutes.
Results
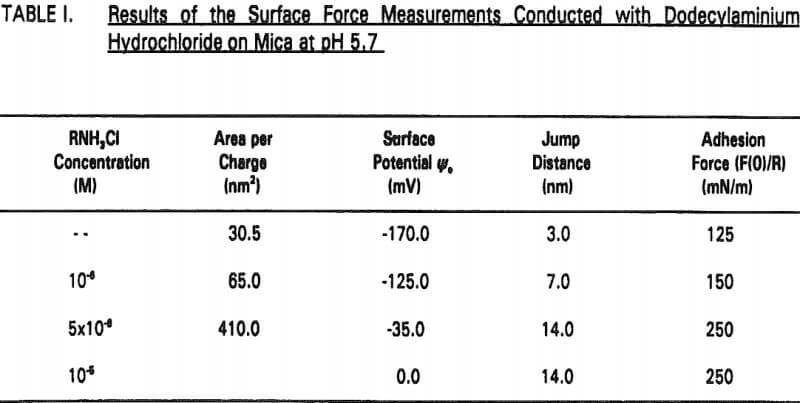
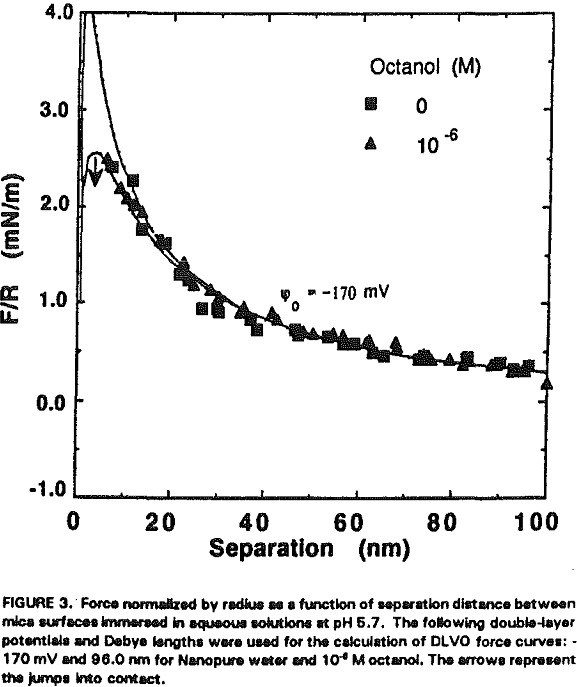
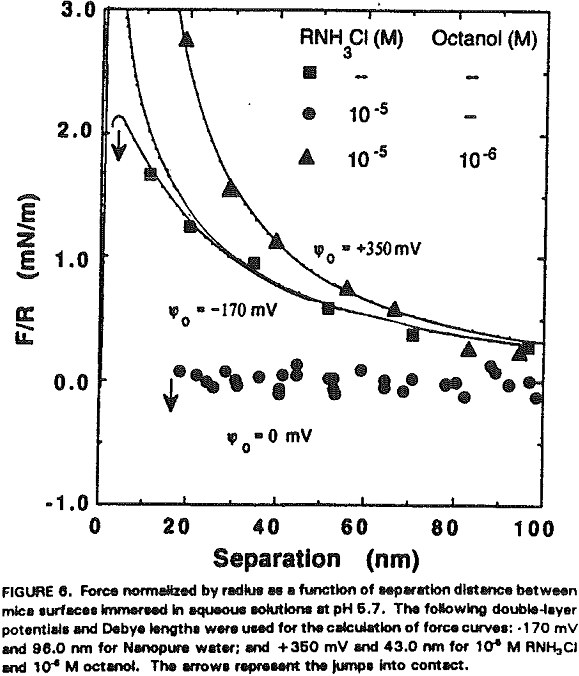
The experimental data obtained in Nanopure water can be best fit with Ψ0 = -170 mV and k¹ = 96 nm. It is shown that the repulsive force decreases with increasing RNH3Cl concentration for two reasons. The first is the charge neutralization by the adsorption of RNH3+ ions on the mica surface. Table I shows the changes in Ψ0 and charge density (given as the area per unit surface charge) at different RNH3Cl concentrations.
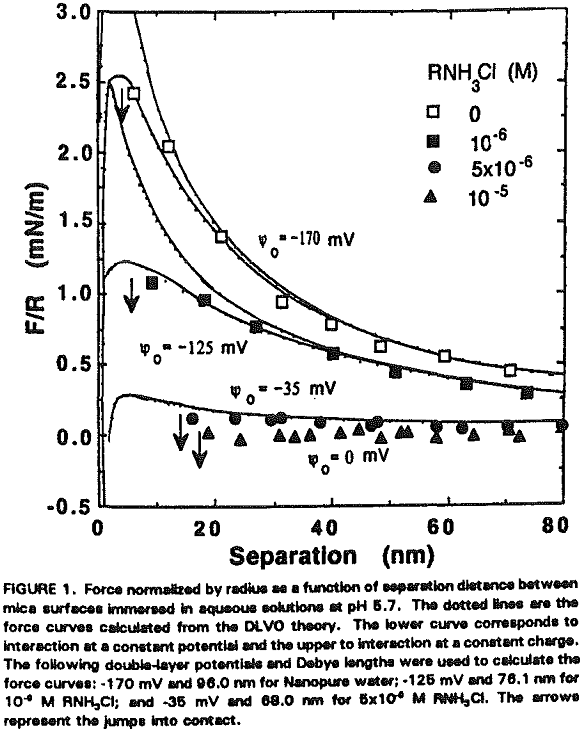
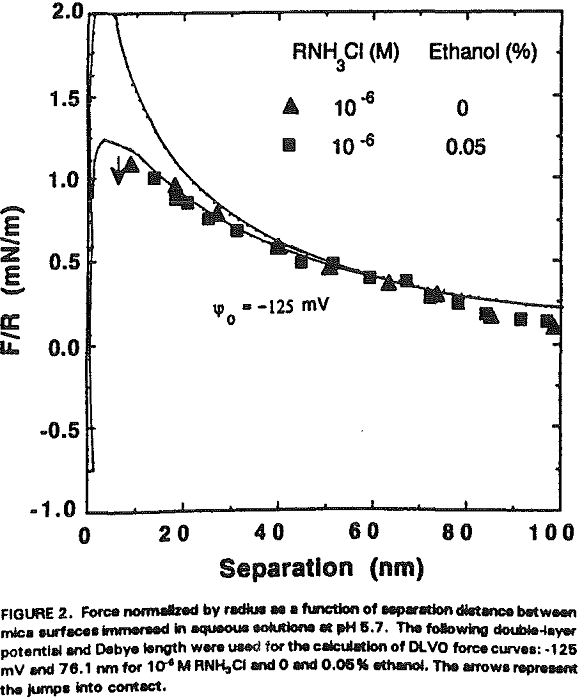
At 10 -5 M RNH3Cl and no octanol, the mica surface is completely neutralized as shown in Figure 6, which is in close agreement with the results of Herder, (1990). In the presence of 10 -6M octanol, however, the repulsive force becomes even greater than in Nanopure water, which may be attributed to charge reversal due to the bilayer formation. The DLVO fit gives Ψ0 = +350 mV and 0.4 nm² of surface area per charge.
Flotation Result
The flotation and surface force measurements were conducted in the present work at pH 5.7, where dodecylamine is completely ionized to RNH3+ ions. Under this condition, the adsorption mechanism should be controlled mainly by columbic attraction.
Both the flotation and surface force experiments conducted in the present work showed that mica and quartz surfaces contacted with RNH3Cl solutions become more hydrophobic when a small amount of octanol is added.
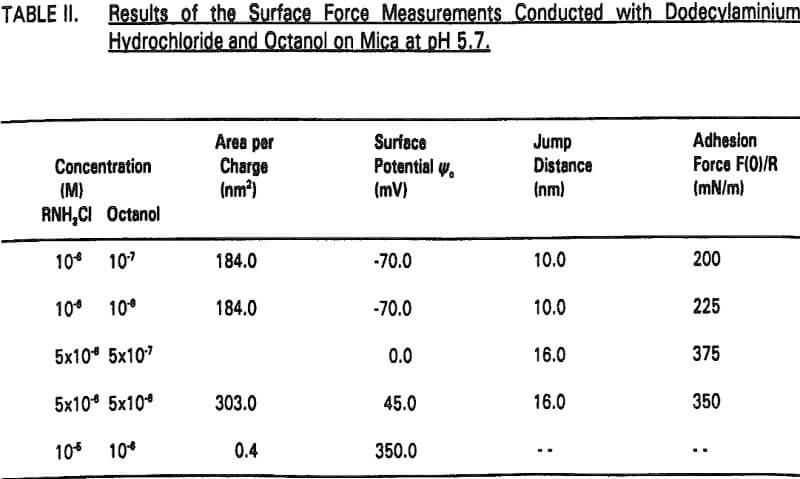
Therefore, the increased hydrophobicity of mica in the presence of octanol may also be attributed to the co adsorption. The increased hydrophobicity is evidenced by the increase in jump distance and adhesion force (Table II).
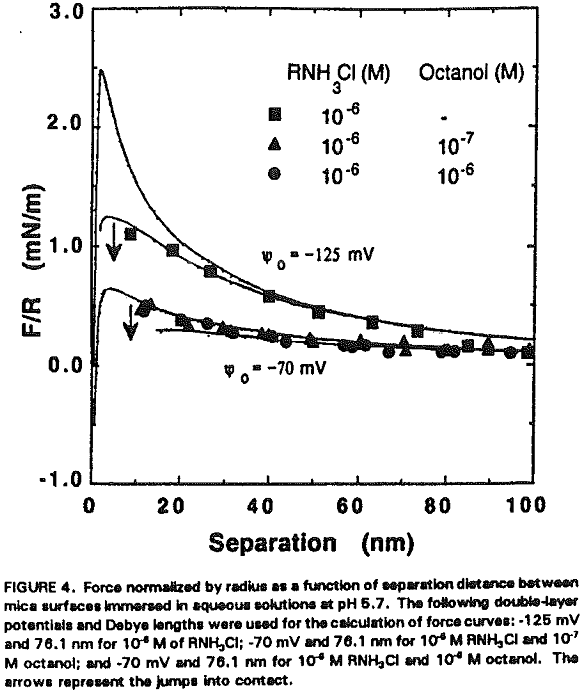
An interesting observation made in the present work is that the coadsorption of octanol not only increases hydrophobicity, but also significantly reduces the double-layer repulsion force. For example, Ψo increases from -125 to -70 mV by the addition of 10 -7- 10 -6 M octanol at 10 -6 M RNH3Cl (Figure 4, Table II).

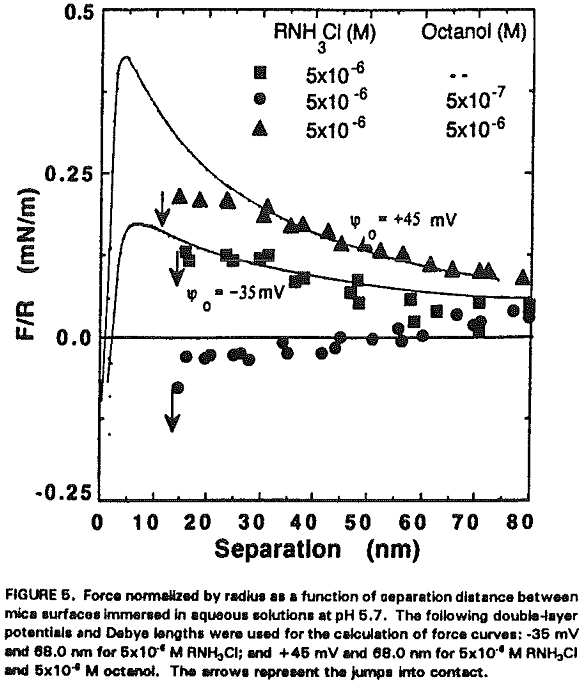
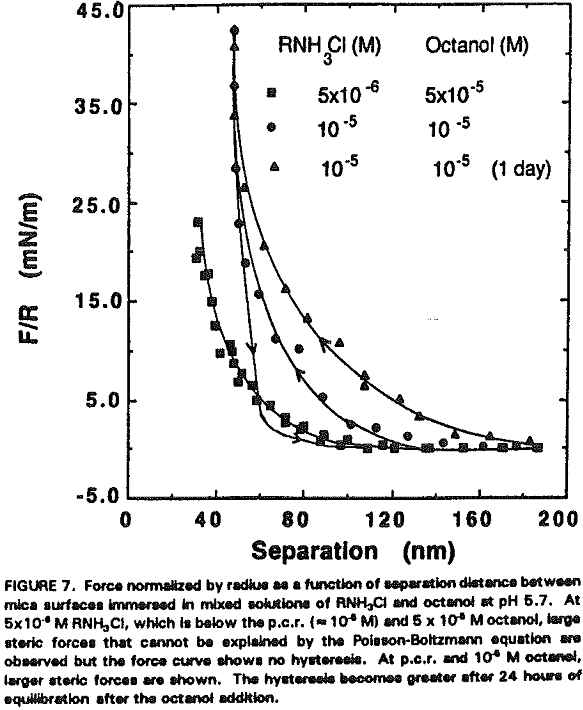
It seems that the major role of octanol in the dodecylamine-mica adsorption system is to decrease the p.c.r. As shown in Figure 5, charge reversal has been observed at 5×10 -6 M RNH3+ (which is below the p.c.r.) in the presence of 5×10 -6 M octanol; the data obtained from the surface force measurements can be fitted by the DLVO theory with Ψ0 = +45 mV. When a smaller amount (10 -6 M) of octanol is added at p.c.r., Ψ0 increases further to + 350 mV as shown in Figure 6.
Assuming that the micellar radius is 1.8 nm, there may be 6-8 layers of micelles on each side of mica plates. If the micelles are of the same size and form a well-ordered structure, the surface force measurements would have shown an oscillation. Since no oscillation has been observed in the present work, the micellar adsorption layer may be considered disordered. It is not likely that the steric force is due to precipitation because the mixed solutions of dodecylamine and octanol were clear.
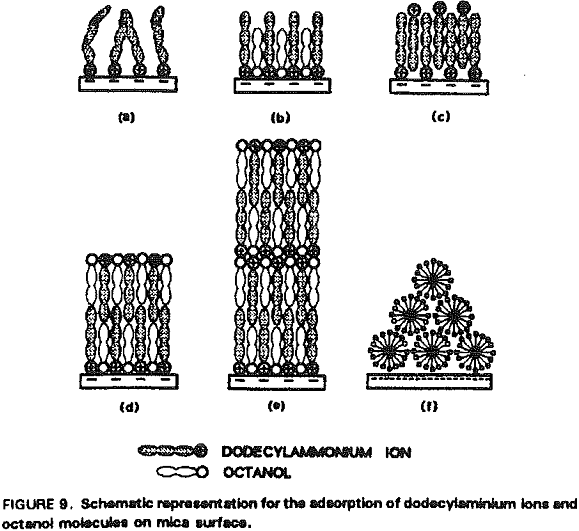
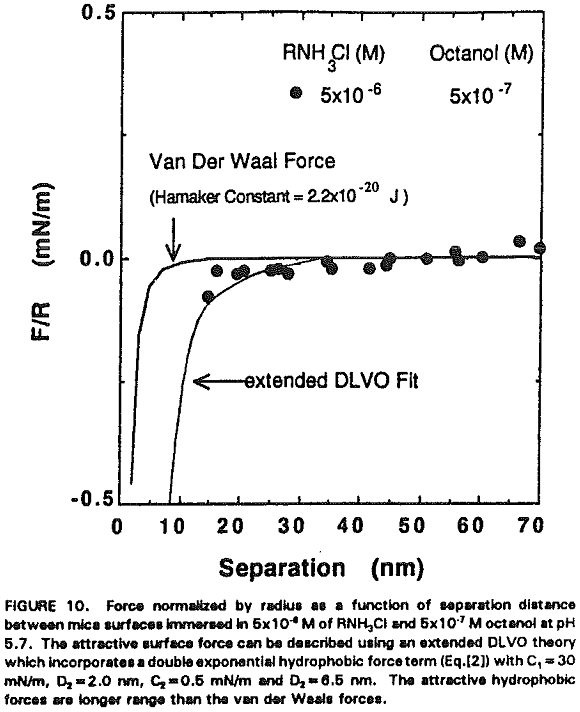
The increased hydrophobicity may mainly be attributed to the higher packing density of the adsorbed hydrocarbon chains in the presence of octanol. Rabinovich et al. (1992) have recently shown that both the decay length (D0) and the pre-exponential constant (C) of insoluble double-chain surfactant- coated surfaces vary significantly with the degree of ordering of the hydrocarbon chains in the monolayer. Figure 10 shows that the surface force measurements obtained at 5×10 -6 M RNH3Cl and 5×10 -7M octanol can be best-fit using the extended DLVO theory, which includes the hydrophobic force term represented by a double exponential function:
F/R = -C1 exp (-H/D1) – C2exp(-H/D2)…………………………………………………[2]
in which C, = 30 mN/m, C2=0.5 mN/m, Dt = 2.0 nm and D2 = 6.5 nm.
In the presence of octanol, the hydrophobicity of dodecylamine-coated mica surface is greatly increased at pH 5.7. This can be explained by a coadsorption mechanism, in which octanol adsorbs in between the dodecylaminium ions adsorbed on the mica surface. In the presence of octanol, the double-layer repulsive force between mica surfaces are further reduced, suggesting that octanol also promotes the adsorption of additional dodecylaminium ions. It is believed that coadsorption increases packing density and ordering of the adsorbed hydrocarbons, both of which contributing to increased hydrophobicity.