Table of Contents
Before treating for arsenic it’s important to understand both arsenic composition and water chemistry. Arsenic (As) is a natural occurring mineral commonly found in clusters paired with sulfur and other heavy metals i.e.: iron (Fe), manganese (Mn), uranium and hydrogen sulfide (H2S).
Recent studies have shown that temperature variance in arsenic prone areas are known to increase arsenic concentrations. While there isn’t enough research to confirm temperature to arsenic correlation, it’s an important observation.
Health Effects of Arsenic
Arsenic is classified as a toxic element and a known carcinogen, prolonged exposure leads to bioaccumulation directly affecting your kidneys, liver, lungs and skin. Due to arsenic reducing the flow of oxygen in your system it may cause cyanosis which is a discoloration of the fingers and toes due to low oxygen circulation, commonly known as Blue Baby Syndrome which affects newborns and patients with a compromised immune system, if not treated may lead to limb amputations.
There is no easy cure for prolonged arsenic poisoning, by the time symptoms are present the focus is on treating the disease and less on remediating the cause.
 Arsenic in Water
Arsenic in Water
Arsenic in water is commonly found in two forms: Trivalent arsenic (arsenic III or arsenite) and pentavalent arsenic (arsenic V or arsenate). While there are others forms of arsenic in mineral form, for water filtration we’ll focus on these two.
- Trivalent arsenic (As III) is non-oxidized arsenic, due to its low molecular weight its harder to filter and more toxic than other forms of arsenic.
- Pentavalent arsenic (As V) is oxidized arsenic, larger in size yet not large enough for mechanical filtration.
Arsenic Guidelines
While there is no safe health limit for arsenic, in 2001 the US Environmental Protection Agency (EPA) proposed lowering the Maximum Contamination Levels (MLC) from 50ppb to 10ppb (0.010ppm = mg/L). The new standard wasn’t official until 2006. While the EPA is responsible for setting MCL national standards, each state can adopt and regulate their own acceptable levels. Some states have advocated lowering the MCL to 5ppb, while these efforts are shared by the EPA it may be too expensive to enforce.
The original MCL was set in 1942 before the EPA was founded in 1970, it wasn’t until the World Health Organization (WHO) lowered the maximum accepted arsenic level that the EPA evaluated the need to reduce it to 10ppb. It is worth mentioning that while the MCL is set at 10ppb it is still not considered a safe level. From the Canadian Water Guideline: “Because arsenic is a human carcinogen, every effort should be made to maintain levels in drinking water as low as reasonably achievable.” (ALARA)
All Public Water Systems (PWS) must comply with the set levels, this includes private wells which supply water to the general public. While homeowners are not enforced to follow the MCL guideline, due to the high risk of developing health problems it is in the public’s best interest to comply.
Arsenic in the USA
The highest concentration of arsenic in the US are found in California, Nevada, New Mexico, New York and Colorado.
The following map shows the arsenic concentration study found by the United States Geological Survey (USGS) in water samples in 2001. Weather patterns have changed in the last 16 years, with extreme drought in California and flooding in Texas which has caused water tables to drop and more often than not, increase arsenic concentrations, which further supports the temperature-to-arsenic concentration theory.
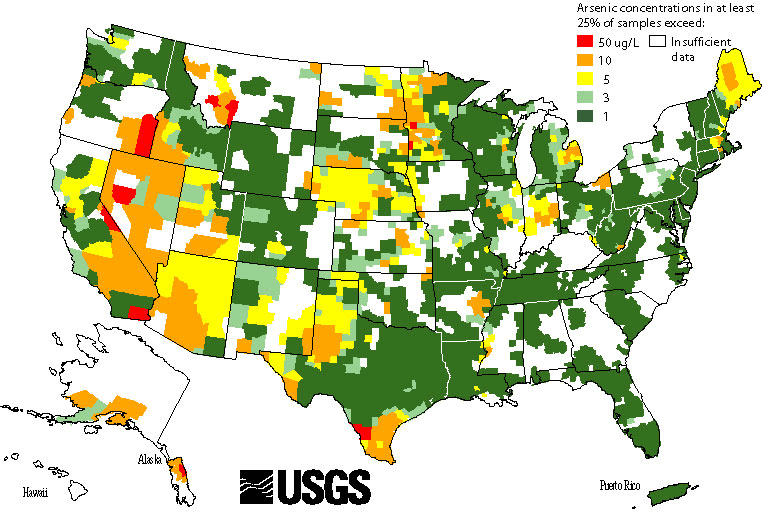
A pilot test run by HP Water in Colorado saw arsenic spiking from 120 ppb to 700+ ppb in a 12 month period with significant temperature changes over a dryer than predicted summer. In a separate project in upstate New York with no significant temperature or precipitation changes arsenic levels went from 5 ppb to 22 ppb in under a year. Which left small water utility providers with hefty infrastructure costs to meet health guidelines.
The Virginia Department of Health (VDH) is one of the first health departments to implement periodic monitoring for arsenic and other contaminants which is reported quarterly on their website. While they don’t recommend a specific treatment they approve applications on a case-by-case basis which can eventually become cumbersome to administer.
Some public utility companies in New Mexico have found it to cost prohibitive to treat arsenic at a municipal level choosing to provide their customer base with point of use arsenic filters rather than implementing city wide treatment.
Arsenic Treatment Methods
The four most common forms of arsenic filtration are:
- Ion-exchange
- Reverse osmosis
- Catalyst media
- Adsorption media
Each one has it’s pros and cons and we’ll cover specific parameters which need to be met for each filtration technology.
Remove Arsenic by Ion-exchange
Ion-exchange is a process of exchanging positive or negative ions with an anion or cation resin, much like a softener it needs a salt brine solution to regenerate and clean the resin bed.
Advantages: Inexpensive media, relatively low contact time needed which translates to higher flow rates with a small footprint.
Disadvantages: Salt regeneration, concentration of arsenic discharge, media fouling can lead to arsenic leaching and competing ions can interfere with arsenic filtration oversaturating the media bed.
Arsenic Removal using Reverse Osmosis
RO systems have become standard when dealing with troublesome contaminants, there are sizing considerations when dealing with reverse osmosis for arsenic treatment
Advantages: Fully scalable to increase flow rates, simple technology, no regeneration cycles.
Disadvantages: Arsenic brine concentration discharge, due to the lower molecular structure of trivalent arsenic (As III) reverse osmosis can merely reduce trivalent arsenic by 20-30% (in controlled lab testing). Large storage tanks are needed for slow RO membrane production.
Catalyst media
The market now supports multiple brands of catalyst media, (Katalox, Filox, Pyrolox, Birm, etc). Catalyst media can only be used when iron is present as it relies on co-precipitation of ferrous to ferric iron to reduce arsenic. It can be used at lower arsenic levels in, the 10-20 ppb range, with an iron ration of 20:1. When lower levels of iron are present, catalyst media can be used to oxidize and reduce arsenic to a manageable concentration level which can then be treated with one of the above technologies.
Advantages: Coprecipitation with ferric-oxide and manganese, no chemical regeneration, longer lasting media.
Disadvantages: Iron and manganese dependant, can only handle lower arsenic levels, concentrated arsenic discharge.
Adsorption Media
What makes adsorption media unique is it’s ability to specifically target heavy metals which is a double edged sword as other competing metals can interfere with arsenic treatment.
Advantages: Longer life compared to ion-exchange, high adsorptive capacity with minimal contact time, no leaching once it reaches it maximum capacity. What makes it different from other arsenic filtration methods is no arsenic discharge, it is all adsorbed in the media.
Disadvantages: Other heavy metals must be pre-treated or they will interfere with the filtration process and saturate the adsorption media. pH dependant; to properly filter out trivalent arsenic (As III) a pH of 8.3 or lower is required.
Arsenic Removal Case Study
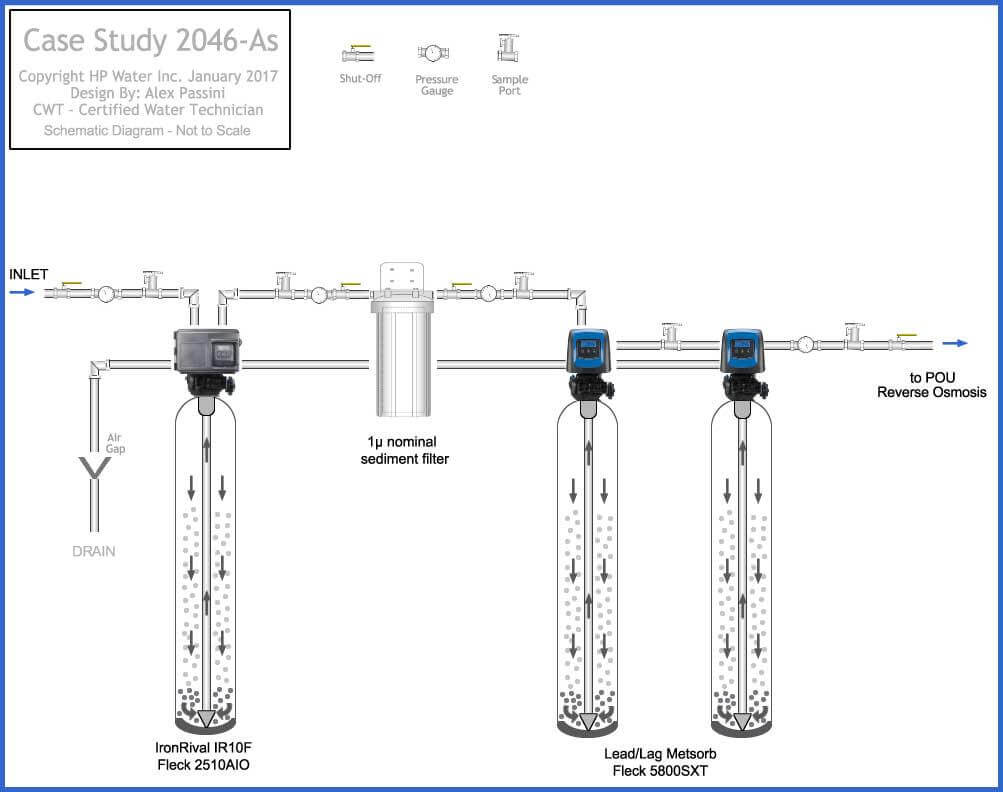
There are cases where multiple forms of treatment can get paired, treatment order is just as important as sizing for volume rather than flow. For a case study in British Columbia, Canada with 1057 ppb arsenic the following was used.
- Stage #1: Catalyst media (filox) paired with an air injection valve (AIO). This method was chosen due to moderate iron levels, while they were below the necessary ratio, they were high enough to be treated and coprecipitated with ferric iron, reducing arsenic by 30%.
- Stage #2: Here we chose to use two lead/lag Metsorb filters (titanium dioxide) with 3 cubic of media. The key to using a lead/lag configuration is maximizing contact time while compounding filtration back-to-back.
- Stage #3: 4-stage reverse osmosis for point of use (POU). We don’t typically choose RO for arsenic filtration due to it’s limitations with trivalent arsenic, however stage #1 oxidized As III to As V which improves arsenic reduction by mechanical process with the reverse osmosis membrane.
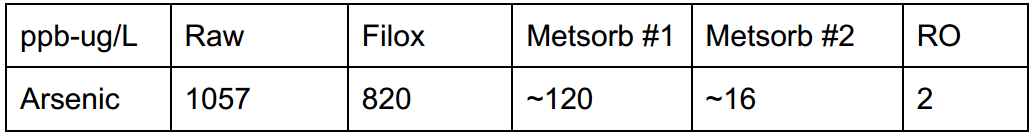
What is the best home system for removing arsenic
Stage #1
