Magnetite Processing & Extraction Jig
This Conkling Jig (ore-concentrating machine or jig), as it is called, is not entirely unknown, having been used at several places with more or less success; but as yet, I believe, no drawings of it or records of its work have found a place in print. Although the jig will be found to be merely […]
Hydrogen Gas
Our Hydrogen Gas experiment involves taking one or two grams of zinc, put them in a test-tube, and add a few drops of dilute sulphuric acid; an effervescence takes place, and bubbles of gas rise through the liquid. Put a lighted taper into the test-tube ; a slight explosion takes place, and you see a momentary flash. […]
Nitrogen Gas
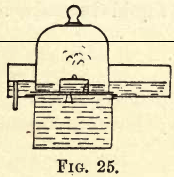
Nitrogen exists in the air, mixed principally with oxygen, so the simplest way to prepare it is to take away the oxygen from the air. Fill the pneumatic trough as before with water, and on the tray stand a bell jar, under which float a small porcelain dish containing a little amorphous phosphorus, as shown in fig. 25. Ignite the […]
Carbon Dioxide Gas
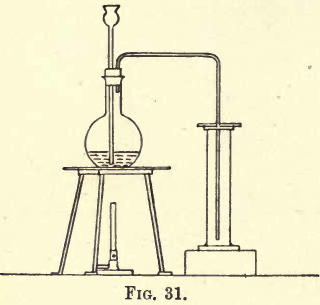
Carbon dioxide is about one and a half times heavier than air, so it can be collected by downward displacement. Fit up a flat-bottomed flask with a thistle funnel and delivery tube, and set up your apparatus as shown in fig. 31. Weigh out about 25 to 30 grams of marble, broken into small pieces, and transfer carefully […]
Nitric Acid Gas
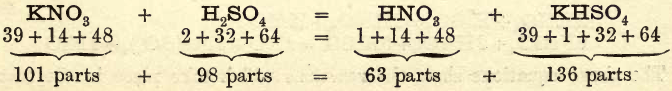
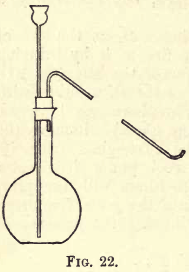
Take a glass-stoppered retort with a capacity of about 250 c.c. and a long beak; clean and dry it. Weigh out 25 to 30 grams of potassium nitrate and transfer it to the retort through either a glass funnel or an improvised paper funnel, taking care that none of the salt adheres to the neck of […]
Nitrogen Monoxide – Nitrous Oxide Gas
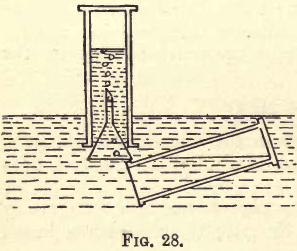
This gas can be prepared from ammonium nitrate, which can be made by neutralising dilute nitric acid with ammonium hydrate. Take some of the nitric acid left over from the last experiments, dilute it with a little water, put it in a porcelain basin, and add ammonium hydrate to it, keeping it stirred with a […]
Ammonia Gas
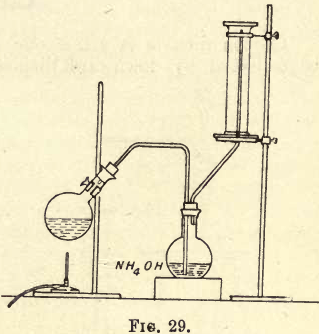
Ammonia Gas is so soluble in water that it cannot be collected as before over water in the pneumatic trough, and as it is lighter than air, fix up your apparatus as shown in fig. 29 and collect it by upward displacement. Mix a little ammonium chloride with an equal amount of quicklime and put it in a […]
Carbon Monoxide Gas

Take a few crystals of oxalic acid and put them into a dry test-tube, then add a few drops of strong sulphuric acid to them and heat gently over a bunsen flame; an effervescence takes place and a gas is given off, which upon applying a lighted taper to the mouth of the tube will […]
Chlorine Gas
Chlorine is a heavy gas, being about 2½ times heavier than air, so that it can be collected by downward displacement. Weigh out about 30 grams of manganese dioxide and about the same quantity of sodium chloride; mix them well together in a mortar, and transfer to a large flat-bottomed flask. Fix up your apparatus […]
Hydrochloric Acid Gas
Our Hydrochloric Acid Gas experiment starts when you place a little sodium chloride in a test-tube and add a drop or two of strong sulphuric acid; an effervescence follows and a gas is given off with a strongly acid pungent odour. NaCl + H2SO4 = NaHSO4 + HCl Set up your apparatus in the same […]
