Table of Contents
- Characteristics Desired in Coke – Chemical
- Blast-Furnace Coke
- Foundry Coke
- Domestic Coke
- Water-Gas Coke
- Characteristics Desired in Coke – Physical
- How to Select Coke making Coals
- Petrographic Analysis
- Direct Determination of the Coke-Making Property
- Plastic Properties
- Determination of Expansion of Coal During Coking
- Summary and Conclusions
Selection of a suitable coal or coals for the manufacture of coke of desired quality with due consideration of the purpose for which the coke is intended requires careful thought, and often it will pay to go to considerable expense in testing such coals as may be available. In general it will always be possible to obtain a satisfactory coal or blend of coals for the particular purpose in view, but in some places the cost of transportation would be prohibitive. It may then be necessary to consider a coal or coals inferior in grade, rank, or both. At times, in fact, only one inferior coal may be available, when the problem becomes mainly one of selecting equipment and operating conditions best-adapted to that coal, keeping in mind that the end product of the plant must compete with the imported product in the market the plant will serve.
This applies particularly to new projects for which it is necessary to build a plant and start with a clean slate, so to speak. However, it will pay every coke-plant manager to employ a force to continually test the coals used and the coke produced to make sure that the quality is not changing appreciably. Furthermore, if the supply of coals used becomes exhausted it will be necessary to use other coals. Continual testing is particularly necessary at plants using borderline coals or blends, where slight changes in the composition or in operation of the ovens may render them dangerously expanding. In this connection it should be stated that control in the newer coke plants is considerably easier than for those built a decade or so ago, consequently in the new ovens a given operating program can be maintained more precisely. This point is important, because one of the main requirements of the coke user is uniformity in both physical and chemical characteristics. For example, any appreciable change in, the quality of coke delivered to the blast-furnace operator will involve changes in operation if the best results are to be obtained. Such changes are expensive; usually they take considerable time and involve production of inferior iron.
Characteristics Desired in Coke – Chemical
It is somewhat difficult to discuss chemical and physical characteristics separately because they are to some extent interdependent; however, it seems self-evident that the kind of coke desired should depend largely on the purpose for which it is intended, and this is true generally. Unfortunately there is no close agreement between users on the properties of the best coke for a given purpose; they even use somewhat different methods of testing as a basis of evaluation. Undoubtedly prejudice influences the choice of users to some extent, but more often it would appear that their choice is based upon experience with a certain coke. They do not want to risk disturbing their operating routine, as the expense of experimenting may outweigh the advantage to be gained in cheapness or better quality of the coke.
According to the principal uses to which it is put coke may be classified as (1) blast-furnace coke, (2) foundry coke, (3) domestic coke, or (4) water-gas coke.
Blast-Furnace Coke
Gluud and Jacobson quote O. Simmersbach on German requirements in blast-furnace coke as follows:

As regards compressive strength, Gluud and Jacobson state they consider this requirement unreasonably high, as in an 82-foot blast furnace the pressure on the coke will not exceed 42.7 pounds a square inch. They conclude from figures available that almost any coke that is suitable in other respects will have a crushing strength high enough for the purpose. Furthermore, the writer has observed that crushing-strength tests or different pieces of coke from the same lot yield widely different results, therefore it is necessary to test a large number of pieces and average the results to insure that the figures are significant.
Rose gives the following chemical properties as desirable in furnace coke:
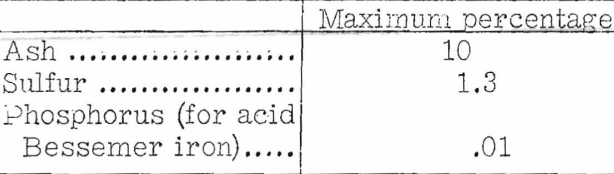
He states, however, that the range of ash content of cokes actually being used is 8 to 16 percent, and that the ash content will depend on the purity of available coals. He calls attention to the importance of uniformity in size and states that sizes larger than 4-inch or smaller than ¾-inch should not be used.
Rose believes that combustibility is an important property in coke affecting blast-furnace operation. That is, the reactivity should be high enough to maintain a small rapid-combustion zone in the bosh and not so high as to cause the carbon dioxide to oxidize excessive amounts of carbon in the upper part of the furnace. This contention is virtually the same as that of Brassert and it seems to be accepted, at least in part, by many blast-furnace operators. Perrott and Kinney analyzed the gases from combustion zones of a number of blast furnaces and found that the oxygen disappeared 24 to 30 inches from the tuyeres and the carbon dioxide at points 32 to 40 inches therefrom. They concluded that some metallic oxides were being reduced by carbon and that factors other than relative combustibility of the coke were responsible for most of the variations in furnace performance.
Mott and Wheeler state that: “It has been argued that a coke of high specific reactivity is desirable to maintain a small oxidation-zone, but at the high temperatures obtaining, the small differences observable amongst commercial cokes are unimportant.” The writer is inclined to favor this point of view and believes, further, that with well-coked charges from byproduct ovens the solution loss will not vary greatly with different cokes considered suitable in other respects.
Foundry Coke
The requirements for foundry coke are somewhat different from those for blast-furnace coke. In the cupola the only function of the coke is to furnish heat to melt the iron, whereas in the blast-furnace the function is twofold – to supply carbon monoxide for reducing ore and heat to melt the iron. In the cupola it is desired to produce a minimum of carbon monoxide because the heat required to form this gas is net available for melting.
Accordingly the coke should have minimum relative combustibility. Also, it should be of large size (over a 3-inch grizzly), hard, and strong enough to prevent excessive degradation by impact of the massive iron charged into the cupola shaft. A coke that degrades excessively in size presents relatively large surfaces for reduction of carbon dioxide to carbon monoxide, involving loss of heating value to the process. Herein may lie the crux of the matter – the logical reason for using large, strong coke. Mott and Wheeler observed “if the combustibility of a coke be defined as its ease of combustion under specified conditions, it will be understood that measures of that property may be misleading. For the ease with which a coke burns is markedly influenced by the conditions under which it is burnt, and the particular conditions of use contemplated may not be comparable with the specified conditions of test.” Thus it may not be so much a question of the intrinsic reactivity of the coke as a question of size and resistance to degradation. Mulcahy stresses the importance of strength in foundry coke and also brings out the point, that a coke that burns too rapidly will make it difficult to maintain the proper height of bed in the cupola. Free oxygen may penetrate above the bed, resulting in oxidized iron.
In regard to the desired chemical properties of foundry coke, one may say that it should contain as small percentages of impurities as possible, due consideration being given the availability of suitable coals from which to make it.
The American Society for Testing Materials states that the following chemical characteristics are desired in foundry coke:
- Volatile matter, not over 2.0 percent-.
- Fixed carbon, not under 88.0 percent.
- Ash, not over 12.0 percent.
- Sulfur, not over 1.0 percent.
As compared with these requirements, those quoted by Gluud and Jacobson are as follows:

It is also specified that there shall be not more than 6 percent of screenings on delivery, that the porosity shall not be over 40 percent, and that the compressive strength shall not be less than 1,420 pounds per square inch.
Domestic Coke
There appear to be no rigid specifications as to the desired chemical composition of domestic coke. The main requirements appear to be that the ash content be as low as possible and its softening temperature be high as possible. Thus Rose states that in some localities it is feasible to specify an ash content as low as 8 to 9 percent, whereas in others cokes containing 10 to 15 percent ash would be considered of fair quality. Ash-softening temperatures of 2,500° F. or higher are desirable, but in some cokes on the market the softening temperature may run as low as 2,300° F. Low- and medium-temperature cokes are preferred for some domestic heating purposes, particularly where it is desirable to maintain a low rate of combustion, as, for example, in the spring and fall. Such cokes are available in some localities in this country and are used to a considerable extent in England. The main chemical characteristics distinguishing them from high-temperature cokes are a higher volatile-matter content (3 to 11 percent) and higher reactivity or combustibility. Probably the most direct small-scale test for relative combustibility of these cokes is that based upon minimum air supply required to keep the fire alight. Ash-content and ash-softening requirements will not differ appreciably from those for high-temperature coke.
Water-Gas Coke
There appears to be little information on the chemical properties of water-gas coke. Rose however, states that “the most serious operating problems connected with water-gas manufacture are due to the presence of clinker, and its removal.” Therefore, he would prefer a low-ash coke (8 to 9 percent) with a softening temperature of about 2,500°. Some operators believe that higher softening temperatures are undesirable – that they increase wall-clinker difficulties. A low sulfur content is desirable, because if the sulfur is high, more of it will pass into the gas and thus increase the cost of purification. The writer has made test cokes from one high-volatile. A coal that is known to produce coke well-adapted to water-gas manufacture. The water gas produced is used in chemical synthesis. The analysis of the coal (as-carbonized basis) and that of the dry coke produced at 900° C. are shown in table 1. Probably the ash and sulfur contents of this coke are about the lowest obtainable. The softening temperature of the ash from the coke is somewhat low. Usually the softening temperatures of the coal and coke ashes do not differ as much as shown. The reason for the difference for this particular coke is believed to be that with the low ash content its softening temperature was lowered to a greater extent by the chance inclusion of iron from the retort in which it was prepared.
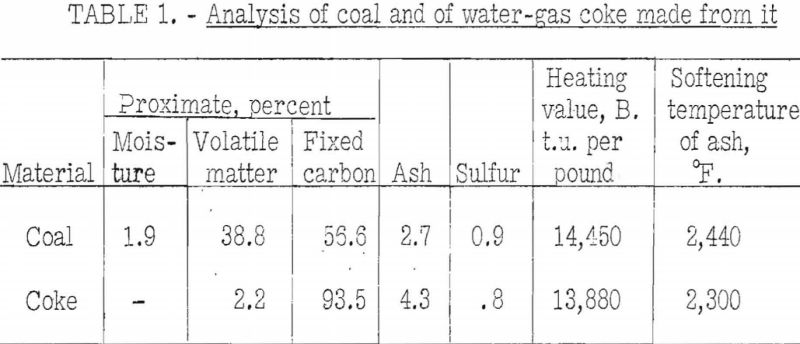
High temperature cokes prepared in the BM-AGA survey always unavoidably contain some iron-scale, and with a small amount of ash its effect is proportionately large. Incidentally, the physical tests on this coke did not indicate it to be as strong or dense as cokes from other coals of high-volatile A rank. Results of physical tests are as follows:

Characteristics Desired in Coke – Physical
The one desirable physical characteristic of coke on which there appears to be general agreement is uniformity in size. This is true regardless of the purpose for which the coke is intended. Producers and consumers alike may disagree as to the size or gradation of sizes best-adapted to a given purpose, but they will agree that the material delivered should be uniform in this respect. Moreover, it is easy to determine this property by a simple screen test once a representative sample is obtained. Most users and consumers will also agree that uniformity in other physical properties is desirable if not imperative, but they are not in agreement as to what the limits in these properties for a given purpose should be or as to standard methods of determining them. The American Society for Testing Materials, Committee D-5, has standardized methods for determination of certain physical properties; but here, again there is no specification as to allowable limits in these properties in coke for the various purposes for which it is burned. The standard methods apply to determination of the following properties:

It is unfortunate that producers and consumers do not use these standardized methods more generally without modification. If they did the comparative value of published data on physical properties would be clear. As it is almost everyone using the methods has introduced at least some slight modification, the effect of which cannot be measured in terms of the A.S.T.M. standard or, for that matter, in terms of any other published standard. The writer believes that it may prove necessary to have modified methods applying only to coke intended for a few specific-purposes. For example, Mulcahy writing on foundry coke, finds he obtains results of greater significance if in the shatter test he determines the amounts remaining on the 4- and 3-inch screens, instead of reporting all sizes over 2 inches as one figure, as in the A.S.T.M. method. With this modification in procedure Mulcahy suggests the following as satisfactory shatter-test figures:
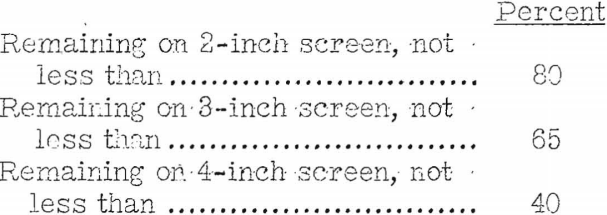
The only figure in the A.S.T.M. shatter-test procedure that can be compared with the above is the percentage remaining on a 2-inch screen. Using the A.S.T.M. method, the writer has found for foundry coke as high as 89.5 for test cokes made in the Bureau’s 18-inch retort and 93.2 for cokes made in the vertical expansion oven both figures applying to percentage remaining on the 2-inch screen. For coke made in the coke oven from the same blend the comparative figure was 83.9. From figures obtained in the same series of tumbler tests the writer suggests that the “stability” factor should be at least 68 percent and the “hardness” factor about 75. The apparent specific gravity probably should be 0.90 or higher.
For all other purposes the strength of coke as measured by the A.S.T.M. tests is not required to be so high. The average 2-inch shatter index of furnace-cokes made from coals of the Appalachian field is approximately 60 percent. Perrott and Kinney found an average of 67 percent for cokes used at eight furnaces in Alabama and Pennsylvania; the high figure was 70 and the low 64 percent. Some of these cokes were made in byproduct and some in beehive ovens. However, practice has changed since Perrott and Kinney did this work; the carbonizing time has been reduced, as well as the quantity of low-volatile used in blends. In some plants high-volatile A coals are carbonized without blending: this is the practice at the Clairton plant of the Carnegie-Illinois Steel Co.; also, the carbonizing time is short. When tested by the Bureau of Mines in 1931, the 2-inch shatter index of coke from this plant was 56.2 percent. At one plant, where coal of higher rank than high volatile B was not available, a 2-inch shatter index of 41 percent was reported. It is reported further that good results are being obtained in the blast furnaces with this coke. From these considerations, it would appear that coals formerly considered very inferior for production of blast furnace coke can now be made to yield good results. No doubt this is due in part to improvements in design of ovens and blast furnaces and in part to improvements in operating technique.
How to Select Coke making Coals
Chemical Analysis
The first step in evaluating a coal for coke making is to obtain a chemical analysis. The proximate analysis, heating value, and sulfur usually are obtained, but often it is advisable to have determinations of oxygen and phosphorus. The softening temperature of the ash is also included where clinkering of the ash is likely to be a factor. The rank of a coal is best-estimated from the proximate analysis and heating value; the proximate and ultimate analyses, with the softening temperature of the ash, fix the grade. For some coke-making purposes the phosphorus content of the coal must be known, and that value can be included with the ultimate analysis. The rank and grade of a coal are among the most important criteria used in assessing its coke-making value, and the analytical data required in their determination are easily and expeditiously obtained in any well-equipped coal analytical laboratory. Furthermore, a wealth of such data, covering coals of known commercial coke-making properties, is to be found in the literature.
Rank
Table 2 shows the range in rank of coals at present considered usable for manufacture of commercial byproduct coke, those of highest rank being low-volatile coals and those of lowest rank being high-volatile C bituminous. As will be seen, the limits of the various classes are fixed for the most part by the fixed carbon content, dry mineral-matter-free basis, plus the moist mineral-matter-free heating value. The volatile matter upon the dry basis is also used where appropriate, and the agglomerating index is used for high-volatile C coals only.
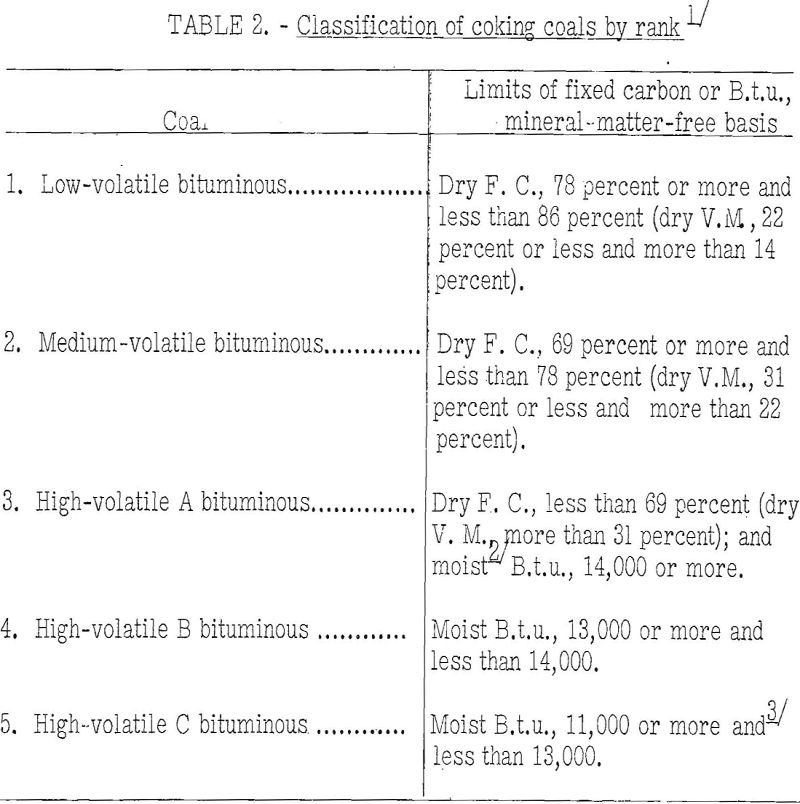
Most coals now used in byproduct ovens are of medium-volatile and high-volatile A rank. Low-volatile coals cannot be coked in byproduct ovens without blending because they expand so much as to damage the oven walls. However, they are valuable for blending with other coals, as, for example, with those of high-volatile A rank, to obtain a mixture of the desired volatile-matter content. The result of blending usually is a more blocky, stronger, and larger coke than that produced from the high-volatile coal alone. The quantity of low-volatile coal used in blends will depend to some extent on the properties of the two coals, but for blast-furnace coke the usual practice is to use 10 to 30 percent of low-volatile coal. For foundry-coke charges 50 percent and even more low-volatile coal has been used, but care must be exercised in operating with such mixes because they are very likely to be dangerously expanding. The only protection against trouble from such blends is continuous use of expansion tests, as will be pointed out further on. High-volatile, B and C coals generally are not used for production of furnace and foundry cokes where coals of higher rank are available at about the same cost. Here again it has been demonstrated—that the coking properties can be improved greatly by blending with 20 to 30 percent low-volatile coal. The writer believes that the possibilities, in this regard have not as yet received the attention they deserve.
Grade
The grade of a coal for coke making is determined by its content of impurities, such as extraneous moisture, ash, sulfur, and sometimes phosphorus, which lower its value. The softening temperature of the ash is of importance in coke for some purposes, the grade rising with this temperature. In general the coke maker will choose the highest-grade coals he can obtain, always keeping in mind the question of relative cost and the importance of obtaining coals of appropriate rank. That is, it may be more economical to sacrifice -somewhat on grade to obtain coals of appropriate rank, and vice versa.
Petrographic Analysis
The petrographic analysis, as made by methods developed by Thiessen and Sprunk gives the amounts of anthraxylon, translucent attritus, opaque attritus, and fusain in the sample in area percent. The method consists in making thin sections, each about ¾-inch square, covering a complete section of the coal bed from top to bottom and measuring the areas of the constituents under a microscope at 200 diameters magnification. The sections are thin enough to transmit light. A summation of the areas of constituents in all the sections gives the quantity of each contained in the coal. Figures 1, 2, and 3 show the appearance of anthraxylon, translucent attritus, and opaque attritus, respectively, as viewed under the microscope; figure 4 shows the structure of fusain at a lower magnification, that is, at 70 diameters. The analysis serves as a basis for classifying coal according to type. The main types in which we are interested here are bright, semisplint, and splint coals. Fusain affects the coking properties of a coal, but it usually is present in small percentages.




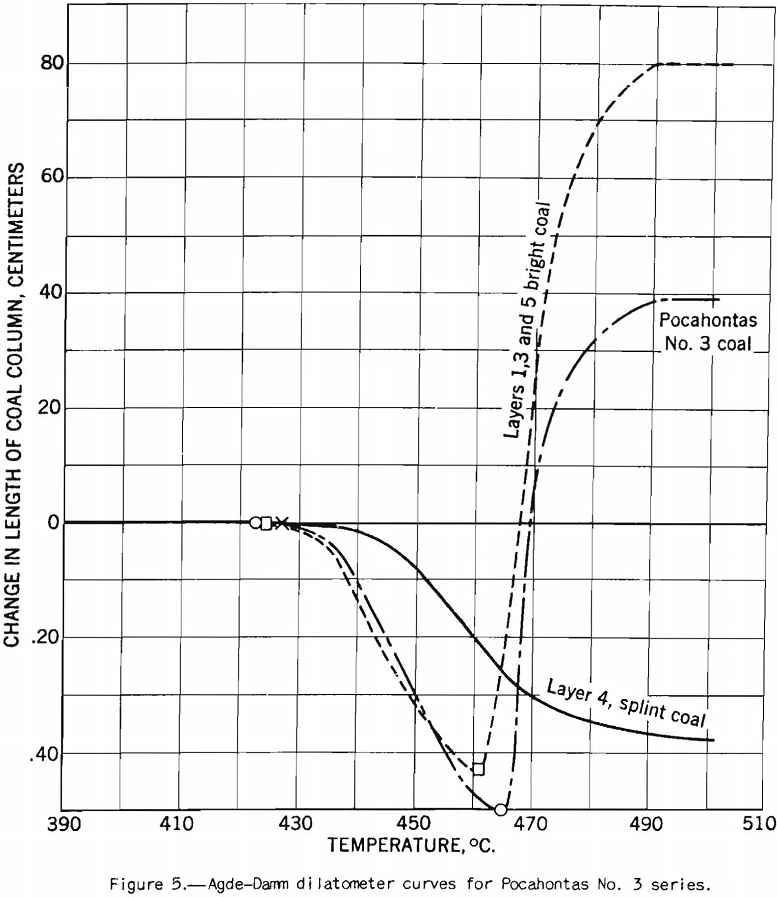
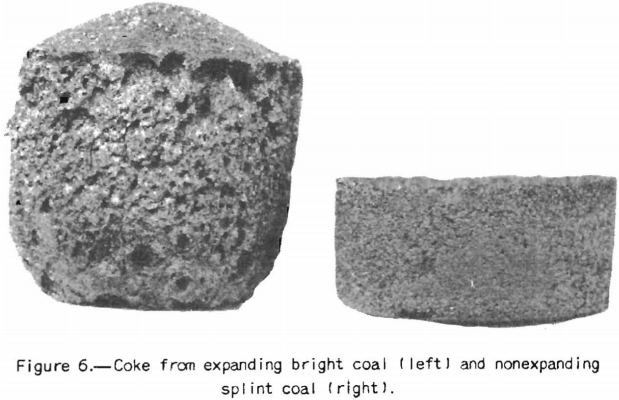
The coal petrographer distinguishes splint coal from bright coal by its greater opacity and. the presence of characteristic large spores. Splint coal may be of slightly higher or slightly lower rank than the rest of the coal in the bed. It is generally less fusible than bright coal and it does not swell, whereas the bright coal is strongly swelling. A further characteristic difference is that the bright coal becomes more fluid in the plastic range than the splint. Figures 5 and 6 will illustrate these points. The agglutinating-value test almost always distinguishes sharply between bright and splint coals the index is much higher for the former than for the latter. The behavior of these types of coal in this test is in harmony with their observed differences in fluidity over the plastic range. That is, the agglutinating-value test consists in determining the amount of dilution with inert a coal will stand and still produce a caked residue on carbonization, or in determining the strength of the caked residue at a given ratio of dilution. Obviously within certain limits the coal that becomes most fluid in the plastic range will stand the greatest dilution by inerts. If, however, one tries to use the agglutinating-value test as a measure of the effect of lowering the volatile content (and fluidity) by addition of the usual percentages of low-volatile coal, one finds that it is not a reliable measure. In fact, examples are on record where addition of as much as 30 percent of low-volatile coal to the high-volatile coal does not affect the agglutinating value at all. The plastic properties, however, vary with the amount of low-volatile coal used; but in blends of high- and low-volatile coals the latter is far from inert so far as coking power is concerned. In fact, low-volatile coal possesses strong coking power, and the purpose in using it is to improve coking power; commercial coke-making experience proves that it does. It is indicated that coke made from some coals is improved by addition of small amounts of carbonaceous inert material such as finely pulverized coke breeze, but that the material added must be fine. The fluidity of the coal in the plastic range is lowered by such additions, but it seems clear that it is not the main factor connected with improvement of coke-making properties. Otherwise stated, lowering the fluidity, per se, is beneficial to some extent, but it is not the main cause of improvement in coking power by blending. The fact that the changes in fluidity brought about are large is mainly incidental; however, it may still be possible to correlate them with changes in properties of the coke. The writer has discussed this phase of the subject at some length to emphasize the importance of knowing how to interpret results of small-scale laboratory tests. That is, we must know their limitations if we are to use them to the best advantage.
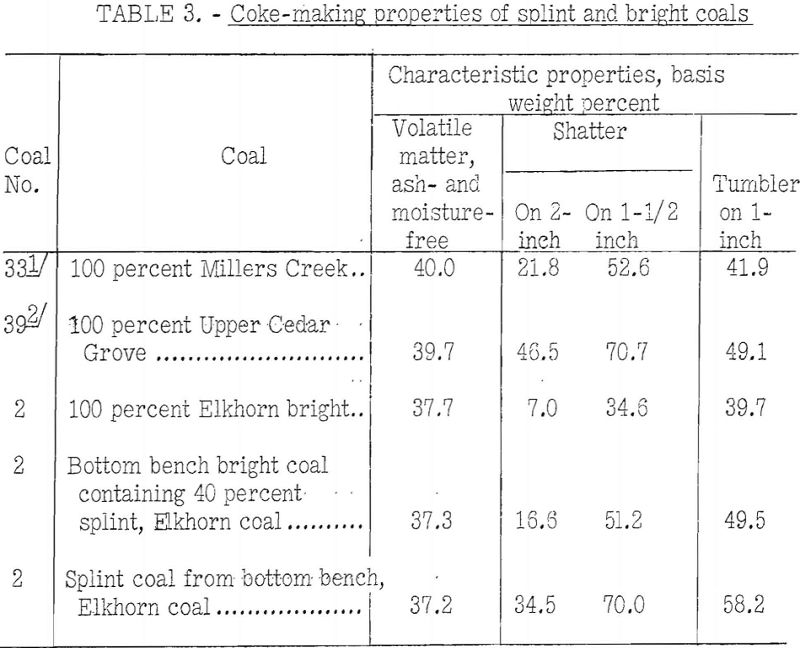
The impression seems to prevail that splint-coal invariably is detrimental to the coke-making properties of coal. This impression may be borne out by coals of higher rank than high-volatile A, but table 3 shows the reverse to be true of two high-volatile A coals on which BM-AGA tests were made by the Bureau of Mines.
The table gives the coking properties of two high-volatile A coals (Millers Creek and Upper Cedar Grove) of approximately the same rank but differing widely in petrographic composition, and similar results for hand-segregated petrographic constituents from the Elkhorn bed.
From the table it may be seen that:
- The Upper Cedar Grove, which is predominantly a splint coal, has much better coking properties than the Millers Creek, which is predominantly bright.
- The splinty samples of Elkhorn show much better coking power than the 100-percent sample of bright coal from the same bed. The rank of all samples is almost the same; however, this is not always true of segregated coal constituents from other coals.
- Tests of the Elkhorn coals were made in the 13-inch retort, whereas all the others were made in the 13-inch retort, for which the carbonizing time was much longer. This fact may have tended to emphasize the differences in carbonizing properties of splint and bright coals for the Elkhorn bed as compared with those found for the other two coals.
Direct Determination of the Coke-Making Property
Full-scale oven tests undoubtedly will give the best information where the type of oven and operating conditions fixed, as, for example, where the operator of a given plant wants to substitute other coals for those he has been using and still please his customers. But even here the method, may not be entirely satisfactory. One operator reported to the writer that he had made a substitution involving one of the coals in a foundry mix, and that as far as he could tell this had improved the quality of the coke. However, cupola operation was affected by the change and the coke was rejected.
Another operator making blast-furnace coke did not change the coal mix but did change to slightly-weathered high-volatile coal. Tests did not show much difference in the properties of the coke, but the blast furnace detected the change, and it was necessary to change operating conditions there. It seems probable that the cupola operator in the first instance cited could have used the coke offered him by changing his operating conditions, but he refused to do it because of the expense of experimenting. It is suggested that the operator making furnace coke might have used the agglutinating-value test advantageously to detect weathering of his coal. It appears to be the best-standardized method available for detecting the extent of weathering of coal. Other drawbacks to the full-scale oven test are its high cost, lack of general applicability of results to other ovens and other operating conditions, and difficulty of precise control of operating conditions. Still it must be conceded that results of such tests are more convincing than those obtained on a smaller scale. It usually is true that the larger the scale of the test the more difficult precise control becomes; on the other hand, it appears equally true that the smaller the scale the less certain one becomes of eliminating the effects of unknown variables or, perhaps better stated, variables the effects of which are imperfectly known. The experienced plant operator ordinarily will first use small-scale tests for which he has a good idea of the limits of application and then make full-scale tests as a last resort if necessary.
Examples of tests made at the coke plant are the box test, small-scale oven tests, and expansion tests. If it is desired to know the yields of byproducts the United States Steel Corporation high-temperature assay is often employed. Some coke plants also use small-scale ovens (about 500-pound charges) heated similarly to a coke oven instead of box tests. An oven of this sort, used to a considerable extent in England, is shown in figure 7. The writer was privileged recently to witness some tests and examine the coke produced in an oven of this type at a plant of the Tennessee Coal & Iron Co. The coke was blocky, though somewhat smaller than that from the ovens; the diameter of the test oven, as the writer recalls, was 12 inches, whereas that of the coke oven was 17 inches. The box-test coke as shown in figure 8 taken from Rose’s paper tends to prismatic shape

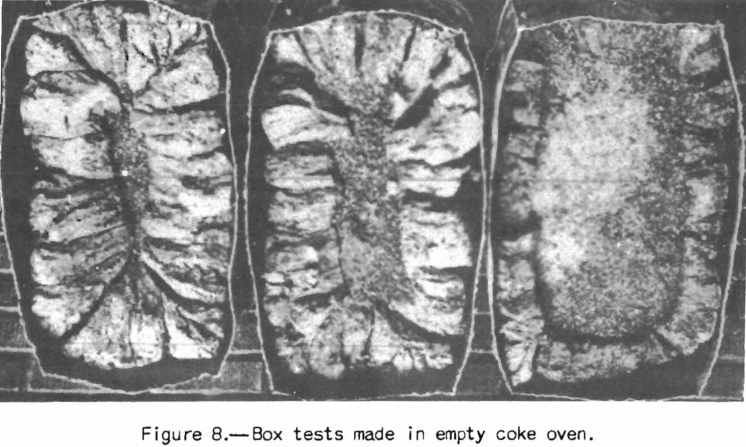
because of conduction of heat along the steel walls of the box. Prismatic shape is characteristic of test cokes made by the Bureau of Mines in cylindrical steel BM-AGA retorts. However, recent tests made in the Bureau of Mines laboratory in cylindrical retorts and coke ovens indicate that the physical properties of the coke can be made to agree closely with those of the oven cokes provided a retort of suitable .size is used. These tests produce coke approaching reasonably close to that from the coke oven.
The Bureau of Mines-American Gas Association carbonization-test method was developed by the Bureau of Mines and the Gas Association in 1929. The main considerations upon which the design was based were:
- The use of charges large enough to yield enough of all the carbonization products to permit of tests of quality ordinarily applied and that the scale be as small as consistent with this requirement. It was realized that, other things being equal, the smaller the scale the less the cost of testing and the more precise the control of carbonizing conditions.
- It was planned to cover the low-, medium-, and high- temperature carbonization ranges in a series of tests on each coal tested.
- It was required that the whole carbonizing and recovery train be virtually gas-tight; the quantities of products being small, it was necessary that leakage be reduced to a minimum so that accurate figures on yields could be obtained.
The retort first used was cylindrical, 13 inches in diameter by 26 inches in height, and provided with a 2-inch outlet for gases and vapors. It is made of 16-gage steel, all seams being welded. After being charged with coal, the disk-shaped cover is fitted into place and the seam is welded. It is then charged into the furnace, previously heated to the desired temperature, and the outlet is connected to the scrubbing train. The temperature at the wall of the retort is kept constant automatically for the duration of the test, the end of the test being indicated by a sudden drop in the rate of gas make. After the test is finished the retort is immediately removed from the furnace, capped, and cooled. It is weighed as soon as cool, and from this weight and that of the retort charged with coal the yield of coke can be calculated. The bottom of the retort is now removed by cutting with a cold chisel, and the coke is removed carefully to avoid breakage.
Samples are taken for physical tests, after which the whole charge, including that used for the physical tests, is assembled, crushed, and sampled for chemical-analysis. All of the products are collected, weighed, measured and analyzed.
The yield and quality of products will agree approximately with those obtained in industrial practice, provided the proper carbonizing temperature is chosen. It should be realized that general agreement with industrial results is impossible because industrial practice varies. It is essential, however, that the test results come within the range of industrial practice, because otherwise the tests might accentuate differences in carbonizing properties of different coals. After all, the main objective is to compare the carbonizing properties of coals rather than to measure them in terms of a 17-inch coke oven working on a 16-hour carbonizing time, for example. Since the work began it has been found advisable to introduce a larger retort (18-inch diameter) for the higher-test temperatures; the quality of the coke from this retort approaches more closely that of the coke oven. A rectangular retort, designed primarily for expansion tests, is available for making blocky coke for comparison with the prismatic coke produced in the cylindrical retort. This retort and the furnace in which it is heated will be described later in this report. Sixty-seven American coals have now been tested; results for the first 30 are given in Monograph 5, referred to above. This publication also gives further details of the methods used.
The coke-plant operator desiring to know the yields of byproducts obtainable from a new coal often uses the Steel Corporation assay. This test is capable of yielding very good results, applicable to a given coke plant under stated operating conditions. It is absolutely essential that the chemist using the test be experienced, and if it is desired to convert the results to those to be expected from the plant he must know the required conversion factors applying to this plant.
In recent years more coke-oven operators than formerly have been using some type of coal-expansion test. The reason for this is that the tendency has been toward the use of blends richer in low-volatile expanding coals. The danger of injury to the coke-oven walls is therefore increased and it is necessary to have and regularly apply some test that will detect changes in expanding properties of coals used.
Plastic Properties
So far, studios of plastic properties of coking coals made in the Bureau of Mines laboratories have not yielded as precise information as we hoped. It is now clear that we were led to expect too much of plastic
studies from claims made for them in the literature. For example, we expected that the expanding properties of the coal would correlate closely with the maximum viscosity in the plastic range. There is correlation, but it is little, if any better than that of the proximate analysis. Probably the main difficulty is that we cannot visualize plastic conditions in a coke oven accurately enough to simulate them closely in the small-scale tests. Some interesting trends, however, have been brought out, and these will be discussed. Perhaps when more data are collected more satisfactory correlation with coke-making practice can be obtained.
Methods of Making Plasticity Tests
The term “plasticity” is used in this connection for want of a better one and because its use was established in the literature before the Bureau of Mines began work on the problem. Consistency or viscosity might apply better, at least over certain parts of the plastic-temperature range.
Agde-Damm Test:
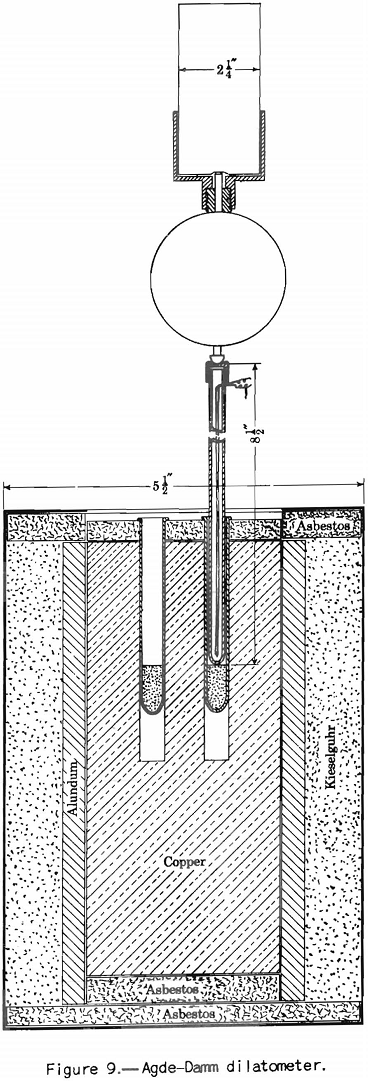
This test probably should be called the dilatometer test; it measures the contraction and expansion of a column of pulverized coal while it is being heated uniformly over the preplastic and plastic ranges. The gases and vapors do not have the same freedom of escape as they do from the plastic zone in layer coking. The writer considers this the main defect of the test from the point of view of practical application. This criticism does not apply to the preplastic range, in which the volume of gases and vapors is small. The apparatus used is illustrated in figure 9. The test consists in heating at a uniform rate a column of coal 0.3 inch in diameter and 0.7 inch in height. The sample is compressed in the tube under a load of 11 pounds. The contraction and expansion are indicated by a special gage to 0.0001 inch as the heating progresses.
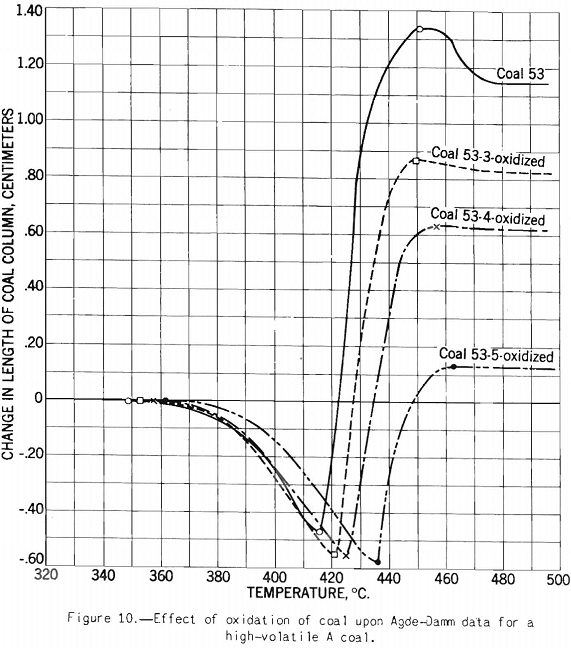
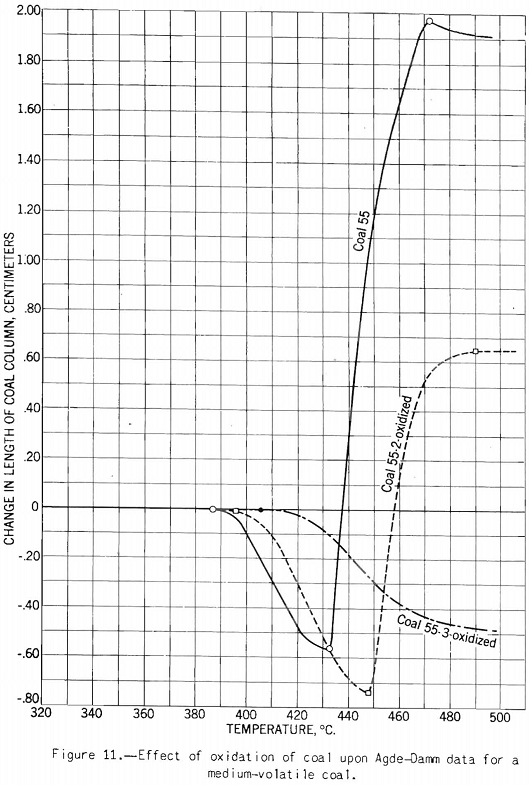
The test indicates the plastic properties of splint and bright coal (fig. 5), the effect of mild oxidation on plastic properties of coking coal (fig. 10), and the effect of blending on plastic properties (fig. 18). Figures 3, 4, and 5 after the test number of the coal indicate progressively the extent of oxidation. It is clear that the test gives a sensitive indication of differences in petrographic composition, of effect of oxidation, and of blending when it is applied to coal from a given bed or different mixtures of the same coals; but it should be stated that the effect as measured is not directly proportional to the change introduced. Figure 11 illustrates the variation in expansion with extent of oxidation for a medium-volatile coal. The amount of contraction under load over the preplastic range is of interest. No other type of plastic test brings out this characteristic so well as far as the writer knows. The cause of the contraction or packing of the charge probably is lubrication of the surfaces of the coal particles by small amounts of oils exuding from the coal while it is being heated over this range of temperature. Such oil wets coal exceptionally well, and no doubt this is a factor that influences packing. It is suggested that this effect found by test and its evident cause offer an explanation for the increase in charge density obtained in coke ovens by Ramsburg and McGurl on slightly oiling the coal charged. If a very small amount of oil, as in the test, produces an appreciable effect it is reasonable to expect that a little more oil added to the coal will increase the effect; and that is exactly what Ramsburg and McGurl found. Other experimenters using the dilatometer method have called attention to contraction found over the preplastic range. It appears to be characteristic of both strongly and poorly coking coals.
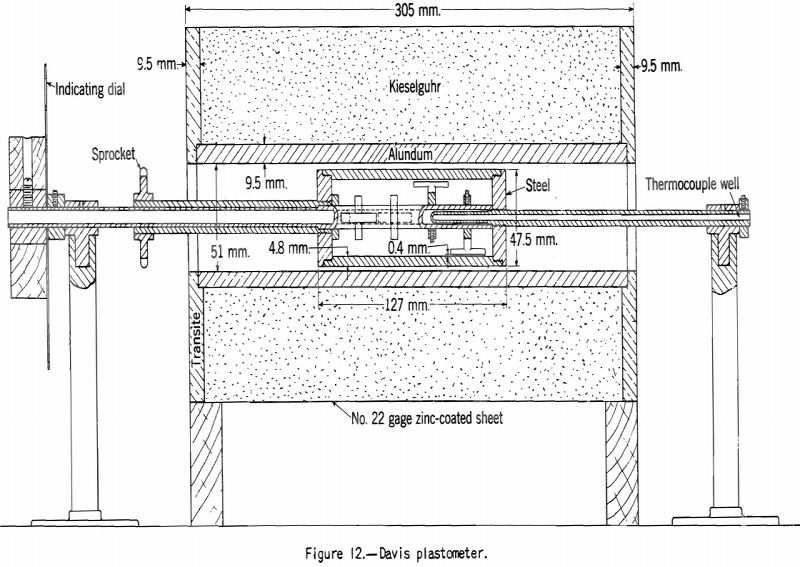
Davis Plastometer:
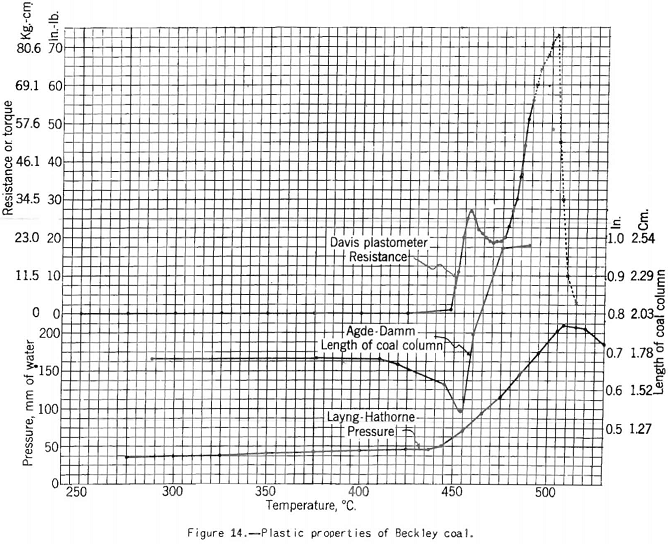
The Davis plastometer (fig. 12) consists of a machined-steel retort that is caused to rotate at constant speed while being heated at a uniform rate over the preplastic and plastic temperature ranges. Inside the retort is a shaft carrying four rabble arms that sweep over the inner surface while the test is in progress. The shaft extends to the outside of the retort through the tubular driveshaft of the latter and is restrained from following the rotation of the retort by springs attached to a graduated head in front of the furnace. Before the coal (18-gram charge) becomes plastic it offers no appreciable resistance to stirring, and the shaft remains stationary. However, as soon as plasticity sets in the springs begin to flex owing to resistance to shear of the plastic mass between the edges of the rabble arms and the inner surface of the retort. The springs are calibrated in terms of pound-inches of resistance, and observations are made of the amount of this resistance and of temperature at intervals over the plastic range. The results of the test are shown best in a plot of resistance against temperature. Figures 13 and 14 respectively, show the types of curves obtained for representative high- and low-volatile coals. There are two peaks of high resistance – one at the beginning of the plastic range and one near the end, the latter being the higher. Between these
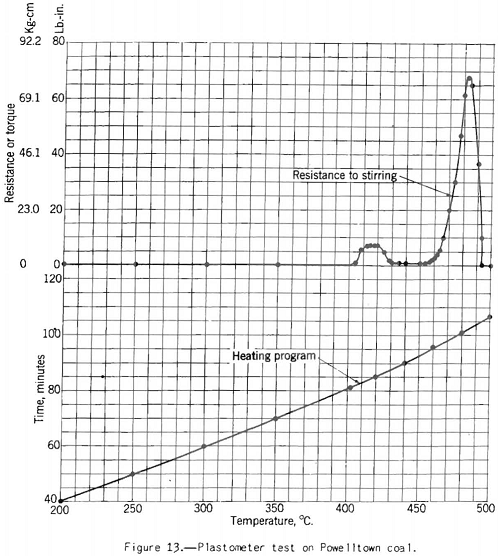
peaks is the range of highest fluidity. This range is shortest and shows the lowest degree of fluidity for the low-volatile coal, If the coal has been preheated or appreciably oxidized the fluidity range is virtually eliminated and the maximum resistance reduced. One can follow the development and changes in plasticity-better in this test than in any other with which the Bureau has experimented. Thus, immediately after the coal begins to fuse the mass is relatively viscous, as shown by the first resistance peak; then as more of the coal substance fuses (or more liquid products collect in the mass) we have a more or less fluid state, depending on the composition of the coal. The last stage in the process is marked by high resistance to shear (high viscosity), and finally the mass sets to form coke. Probably at this point it pulverizes; at any rate, it suddenly offers no more resistance to shear than the unfused coal at the beginning of the test.
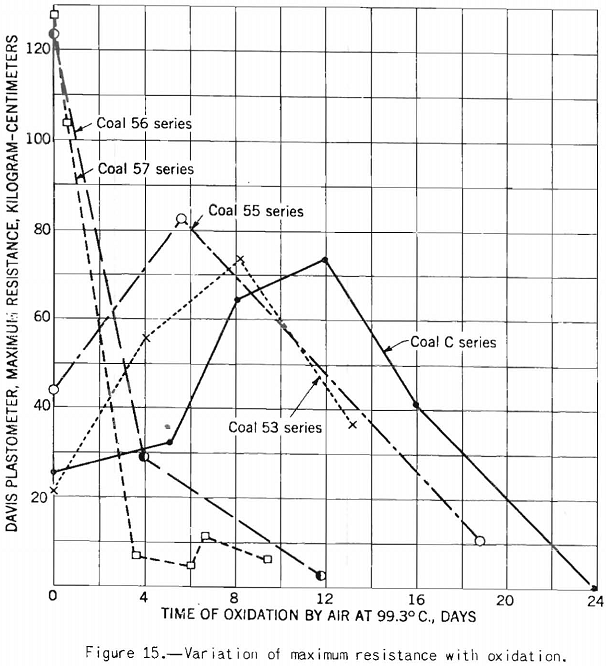
Figure 15 shows that the test is sensitive enough to detect oxidation.
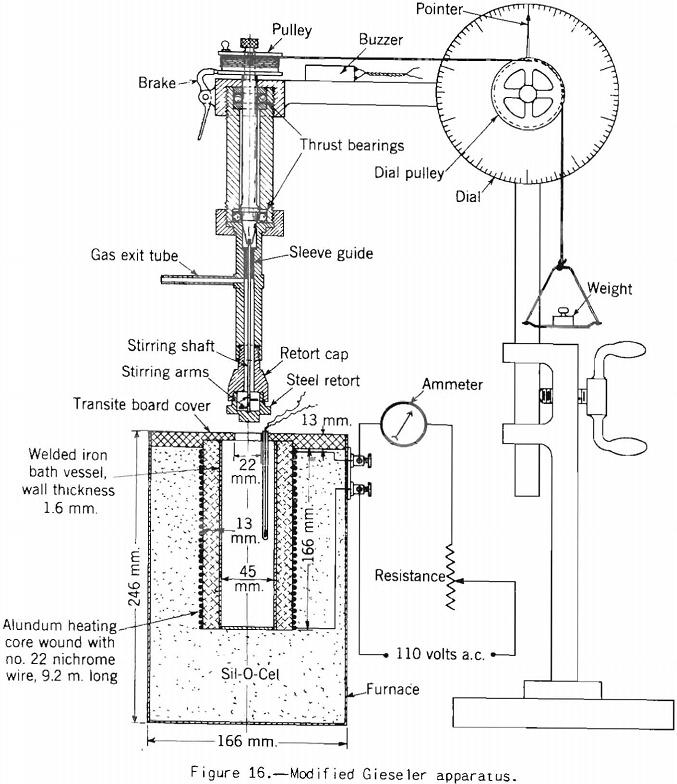
Gieseler Test:
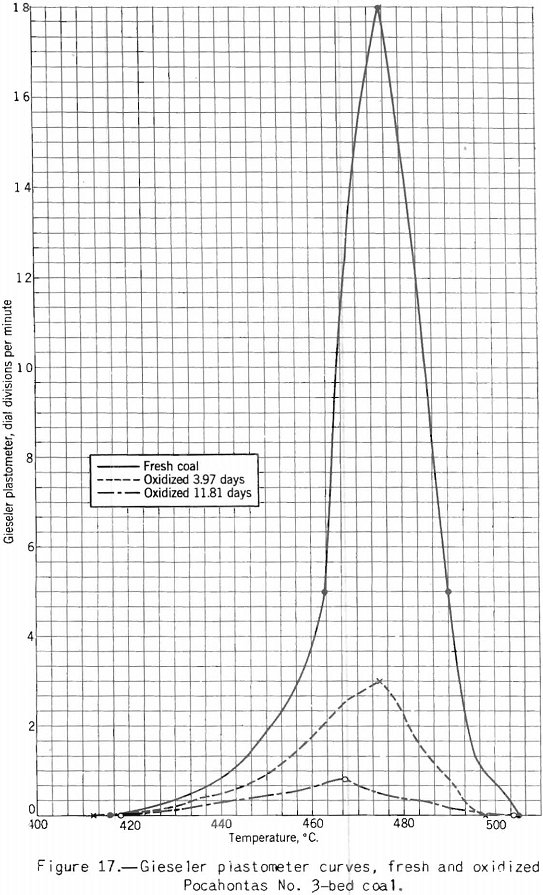
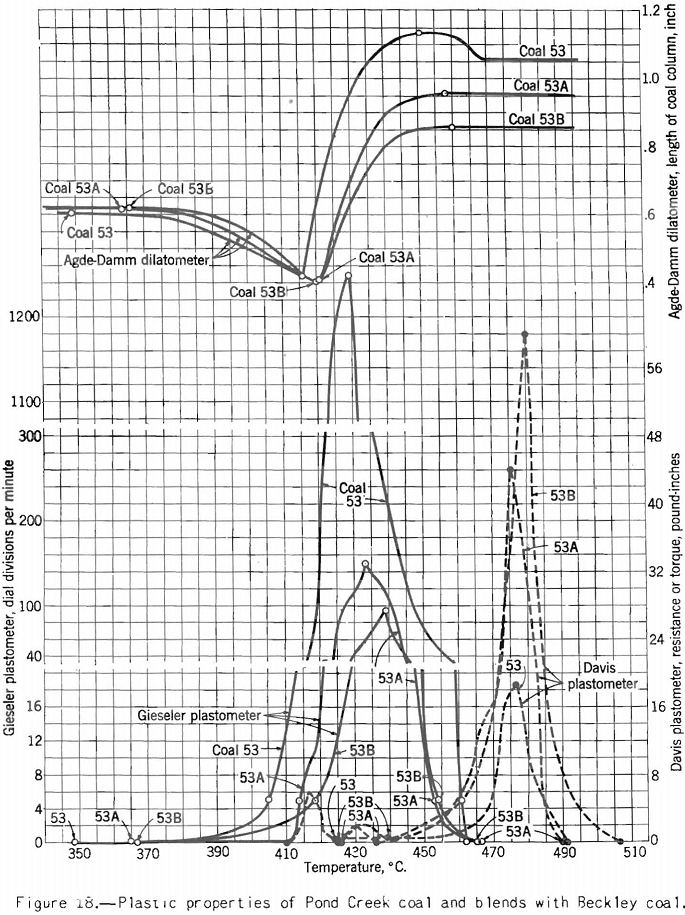
Apparatus for making this test, originally developed by Gieseler is shown in figure 13 as modified by the Bureau of Mines. The method of making the test consists in observing the rotation of a special stirrer in a small charge of fine coal while it is heated at a constant rate over the plastic range. Rotation of the stirrer is brought about by a weight attached to a string passing over the pulley attached to a pointer moving in front of a circular scale, as shown. The string is fixed to the stirrer pulley and wound around it. When the heated mass of coal begins to fuse the pulley begins to rotate; and the higher the fluidity developed, the more rapid the rotation. A plot of the rate of rotation against the temperature shows the changes in fluidity. Figure 17 shows how the fluidity by this test varies with oxidation of Pocahontas coal, and figure 18 shows the effect on fluidity of blending high-volatile Pond Creek coal with 20 and 30 percent of low-volatile Beckley (53A and 53B, respectively). The figure also shows how the indications of the three plastic-range tests used by the Bureau of Mines are related. It may be pointed out that:
- The high-volatile coal becomes the most fluid and swells the most in the Agde-Damm test; it also gives the lowest maximum resistance in the Davis plastometer.
- The effect of raising the rank of the charges by adding low-volatile coal is to decrease the swelling and fluidity and increase the resistance to shear at its maximum.
- The temperatures at which swelling begins correspond very closely to those marking the beginning of resistance to shear, but the point, of maximum fluidity comes slightly earlier (lower temperature) by the Gieseler-plastometer than by the Davis method.
- The swelling test (Agde-Damm) gives no indication of maximum fluidity, no doubt because the evolution of decomposition products (cause of swelling) at this point is so rapid as to mask any effect on the test maximum fluidity might have on the curves.
- When the charge ceases to swell, the fluidity drops almost to zero. Moreover, the resistance to shear rises sharply (the mass becomes more viscous) toward its final peak. It can be shown that at this temperature gas is being given off at a rapid rate; in fact, it is about constant at its maximum rate for the plastic range from this point to the end. One might argue that at a point near the end of the plastic range we have the optimum conditions for development of plastic pressure, and therefore expansion of the plastic zone, because here the rate of evolution of gas is at a maximum and the resistance offered to its escape is highest. This argument is roughly borne out, because coals of medium- to low-volatile rank develop the highest, resistance to shear, and they yield almost as much gas (volume basis) as higher-volatile coals. It is also true that coals in this range of rank produce the strongest cokes.
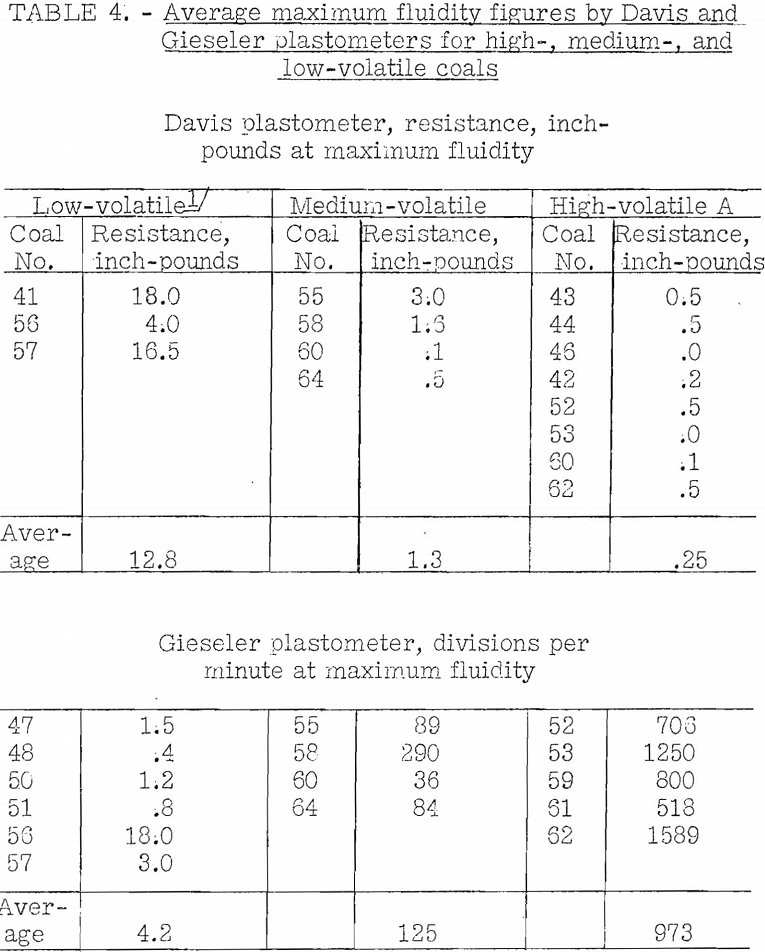
Table 4 gives some average results of measurement of maximum fluidity on a number of coals of different ranks by the Gieseler and Davis plastometers. It is shown that the Davis plastometer indicates variation in maximum fluidity with rank of the coal, as does the Gieseler, the latter being the more sensitive. The results vary widely in each range of rank, and this may accord with what should be expected where the indications are large. Erratic results are to be expected for the Davis plastometer in the low-volatile range because here the plastic curves are likely not to be well-defined, unless the heating rate is more rapid than normal. Some low-volatile coals will not coke at all in the BM-AGA carbonization tests at low temperatures where the rate of coking is very slow. Furthermore, with high-volatile coals where the minimum resistance by the Davis plastometer is very low (high fluidity), variations found between different coals are not significant. This plastometer does not give a precise measure of the degree of fluidity; however, it does give a measure of the length of the fluid range.
From what has been said it will be clear that no one of the plastic-range, tests alone will give a precise measure of the quality of coke to be expected from a given coal or blend and hence serve as an accurate guide in the selection of coals for coke making. It seems doubtful if combined results of several tests will satisfy this rigid requirement. However, the results, taken together, do serve to give one a picture of what happens in the plastic range of temperatures where the coke is being formed; and any means that will do that, even if the picture is imperfect, is worth while.
Determination of Expansion of Coal During Coking
Development of a thoroughly reliable test for this purpose is extremely important, not as a guide as to what coals to select for byproduct coking but rather as a guide to what coals not to select. Expanding coals may well exert enough pressure on the walls of the coke ovens to damage them seriously. They may even be only slightly expanding and still do considerable damage in time. Moreover, with some expanding coals, at least, damage may be done long before the operator suspects that anything is wrong. The highest pressures are exerted toward the end of the coking period, after which the charge contracts and the operator may experience no difficulty in pushing.
According to Brown who has made a large number of expansion tests on a wide variety of coals, the dangerously expanding coals arc in the low- and medium-volatile ranges of rank and in blends of coals falling within these ranges. He found that the low-volatile coals expand the most but that expansion is not directly proportional to the rank of the coal or blend, particularly in the medium-volatile range. This is an important finding because the best coals for byproduct coking are of medium volatile rank.
It is exceedingly difficult to design a test to determine whether or not a coal is dangerously expanding, particularly if it is on the borderline between dangerous and safe coals, In the first place, walls of different makes of ovens differ as to the pressures they will withstand without damage, and in no design is there definite information as to the magnitude of dangerous pressures. Moreover, pressures will vary-with operating conditions, bulk density of the charge, carbonizing time, etc., so that, for a test to have greatest practical value, it must be flexible enough to enable one to simulate carbonizing conditions of the coke oven to which the result is expected to apply.
The first test oven built by the Bureau of Mines followed closely the design of the first sole-heated oven used by Brown. The oven is shown in figure 19. In it a charge of about 40 pounds of coal is heated electrically from the bottom under a load of 2.2 pounds a square inch, and the expansion is followed by a cathetometer as coking progresses. A number of coals
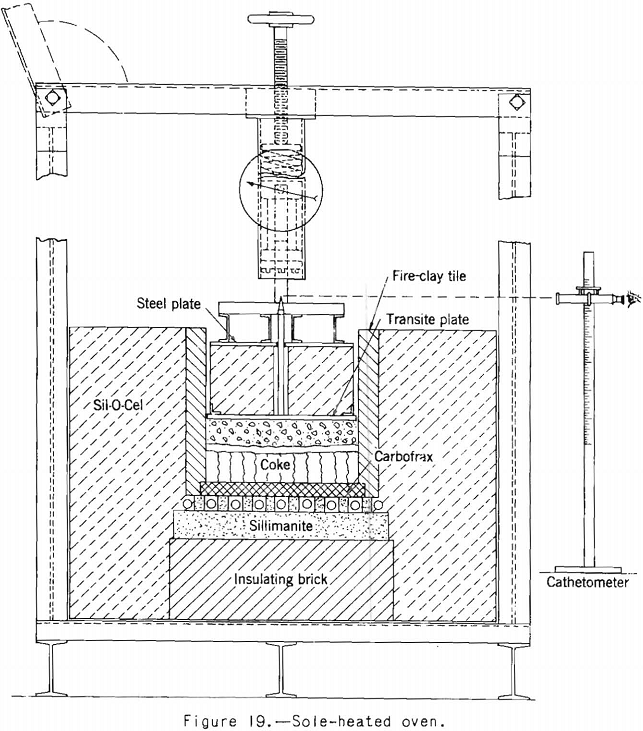
have been tested, and fair agreement with Brown’s test results on the same coals has been obtained. In the sole-heated oven, the loaded cover on top of the coal charge almost entirely covers the charge, a condition that does not simulate that in a coke oven, where the top of the charge is free to expand into the free oven space above.
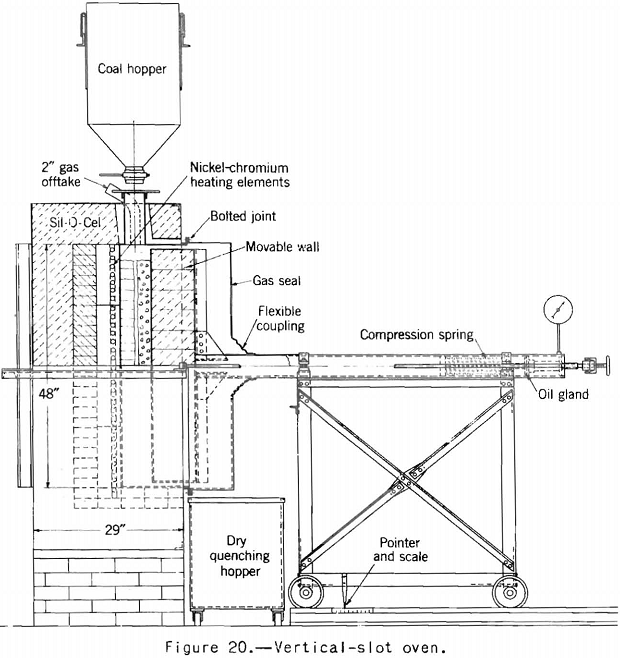
Accordingly, the vertical-slot oven shown in figure 20 was built to determine if expansion into the free space was an important factor. The results obtained in this oven agreed fairly well with those obtained in the sole-heated oven, so it was decided that the factor could not have much importance. Results of tests by the two ovens on the same group of coals are given in table 5.
One-sided heating as used in these two ovens has one and possibly two defects. The charge is not thoroughly coked through to the maximum temperature, and meeting of two plastic layers, as in the center of a coke oven, does not occur. It is not possible to heat the part of the charge adjacent to the cold-wall much above 700° C. without overheating that next to the hot wall, and so the maximum contraction of the coked charge cannot be realized. These defects do not necessarily invalidate one-sided heating tests for plant control. Brown’s work indicates that the expanding properties of coals in the small test are proportional to the oven-expanding properties and that the test is a sensitive indication of changes in the coals. Altieri’s results indicate the same thing. However, the writer believes that if one is interested in estimating the expanding properties of a given coal under different oven-coking conditions one must simulate the different oven-coking conditions in the test as closely as possible. This requirement would appear to call for heating from both sides. Furthermore when one is interested in a survey of the coking properties of coals as is the Bureau of Mines, it is desirable to know as much as possible about it. It may be that when enough data are collected by both methods we will find the results correlating closely enough for all practical purposes, but we do not now know how closely they will correlate.
Koppers and Jenkner built a large-scale, slot-type, expansion oven that was heated from both sides, as in a coke oven. They found that the maximum expansion pressure occurred shortly before the end of the coking period when the two plastic layers met. Russell recently obtained results in a vertical slot oven similar to that of Koppers and Jenkner that were in substantial agreement with those obtained by these investigators. He heated a charge 12 inches thick from both sides and obtained a peak pressure of about 6 pounds a square inch when the two plastic layers met at the center of the charge. He then heated a charge 6 inches thick in the same oven from one side only but otherwise maintained the same carbonizing conditions, the maximum pressure obtained being about 4.3 pounds per square inch. Figure 21, taken from Russell’s paper, is a plot of his results. Beckley-bed coal was used in 100-percent charges, and this coal so charged in a coke oven would be considered dangerous.
The Bureau of Mines has modified the vertical-slot oven shown in figure 20 to permit heating from both sides. Briefly, modification consisted in installation of electric heating elements in the movable wall, installation of a thermoelectric controller to control the temperatures of both walls, dispensing with the gas seal and flexible coupling, and

substitution of 20-gage welded-steel retorts for the one having the side adjacent to the movable wall open as originally used. Closed steel retorts were adopted because, where metallic electric heating elements are used, it is absolutely necessary to protect them from distillation gases and vapors; otherwise their life will be short. However, the introduction of a closed retort necessitated a change in the method of operation; that is, with the one-sided heating one can work at constant volume and measure the amount of expansion, whereas with a retort closed on all sides it is necessary to work at constant volume and measure the pressure. Otherwise the resistance offered by the wall of the retort to expansion will be a factor difficult to evaluate. The thermoelectric controller is fitted with a device for changing the heating rates at the walls to simulate those at the coke-oven walls. To do this it was necessary to provide an extra synchronous motor similar to that used in the controller-recorder to advance the contact points of the controller uniformly with respect to time but in accordance with any rate of temperature rise desired. The principle of the arrangement is shown in figure 22. The device was worked out by H. S. Auvil of the Bureau of Mines staff. It will be seen that, to use it, sheet-metal curves following the countours of the various time-temperature curves desired and the instrument will automatically reproduce these temperature programs at the oven walls.
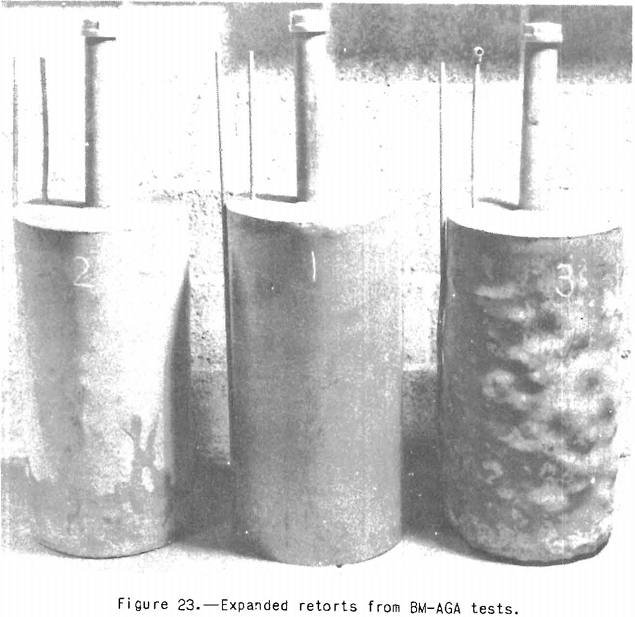
Reynolds and Birge investigated, the pressures developed during carbonization on the coal side of the plastic seal in Bureau of Mines-American Gas Association retorts. They found high pressures of the order of 100 pounds a square inch for low-volatile highly expanding coals, and low pressures (less than 1 pound a square inch) for high-volatile A coals. This result is understandable when we remember that the viscosity of the plastic mass is considerably higher, by plastic range tests, for the low-volatile than for the high-volatile coals. If the viscosity is high, obviously the plastic layer will be less permeable to gases and vapors. Maximum pressures are developed in all instances in which the charge is about 70 percent carbonized. Reynolds and Birge also found that coals developing high pressures behind the plastic, seal deformed the steel retorts and further that such coals are known to be expanding in coking practice. It is not suggested that the total pressure behind the plastic seal is transmitted to the walls of the retort, although it may exert some influence on the effective pressure at the walls. We do know, however, that the permeability of the plastic layer is low, and therefore gases generated within the mass will tend to distend it. Furthermore, tests in which the pressure behind the plastic seal was released, though not entirely satisfactory, indicated that the retorts were still deformed to about.the same extent. It is easy to measure the extent of deforma-deformation by immersing the retort in a known volume of water before and after the test and noting the volumes of water displaced. This is being done in tests where appreciable deformation is detected. Figure 23 shows three retorts, No. 1 being one in which no tests have been made, No. 2 showing deformation caused by a borderline coal, and No. 3 showing deformation caused by a highly expanding coal. No. 2 is rated as a borderline coal, because it is known to give trouble in ovens under some operating conditions and not under others. It would therefore appear that the BM-AGA test is a sensitive measure of the practical expanding properties; however more comparative data will be required to prove or disprove this point satisfactorily. For the present, it probably is best to handle with caution coals that even slightly deform the BM-AGA retorts. Another point of interest in this connection is that the contour of the bumps on the expanded retorts is the same as that of the cauliflower ends of the pieces of coke. The curvature is greater for tills cylindrical retort than for a coke oven, because oven coke is blocky whereas that from the cylindrical retort is prismatic, but the curvature is common to both coke shapes. It therefore seems clear that maximum expansion pressure on the walls of the retort or oven is exerted at the center of the cauliflower ends of the coke. The writer believes that pressure thus exerted at a number of points, so to speak, may cause more damage to oven walls than would occur if the pressure were exerted uniformly over the whole surface. Most investigators apparently believe that expansion pressure is caused by distention of the plastic mass by gases confined therein and that the thicker and more viscous the mass, the greater will be the pressure, other things being equal. The writer holds this opinion. It is supported by the fact that in the pre-plastic range there is only net contraction, and,the same is true of the coke over the range 500° C. to maximum oven temperatures.
Summary and Conclusions
The paper discusses chemical and physical properties desired in cokes for the several main uses to which they are put and then deals with test methods available for determining the suitability of coals for making the cokes. It is possible to obtain, at least in the United States, coals eminently suited to any given purpose; but there are localities where only inferior coals may be obtained, except at prohibitive cost. Even in such instances the end product of the industry – steel, for example – in which the coke is used may be in such demand that it is feasible to produce the coke from coals available. It may be concluded that:
- One of the main requirements of coke users is that the coke delivered to them be uniform both as to chemical and physical properties. Changes in the properties of the coke may involve changes in the operation of user’s equipment, and such changes mean loss of time, money, and material.
- Authorities are not in close agreement as to desired properties in cokes for the several purposes and they do not consistently employ standardized methods for testing the cokes, although standardized methods are available. The difficulty in fixing the properties desired in coke by rigid specifications arises, at least in part, from the fact that in some paces only inferior coals are available. Even so, the use of coke from such coals may be economically feasible.
- Probably the most satisfactory method of determining the suitability of a coal for making coke of required quality is to make a full-scale oven test in which are maintained the carbonizing conditions under which it is proposed to use the coal. The coke-plant operator ordinarily will use this method as a final check on results of small-oven tests, box tests, and the like.
- It is necessary for plant operators using borderline coals (or coals that expand or contract only slightly) to make expansion tests as a matter of routine, since the coal being delivered may change enough to cause damage to oven walls. It is believed that the moderate-scale expansion-oven test, such as the sole-heated oven tests, are sensitive enough to afford protection in that regard.
- Plastic-range tests are of value in studying effects of blending, weathering, and petrographic composition on coking properties, but in the present stage of their development they are not adequate to show precisely the quality of coke to be expected.
