Table of Contents
- Fabrication of Bio-Fix Beads
- Identification of Bead Fabrication Parameters
- Selection of Biomass
- Standard Bead Fabrication Procedure
- Bio-Fix Bead Sorption and Elution Characteristics
- Metal Sorption from Dilute Waste Waters
- Metal Sorption Kinetics
- Effect of pH on Metal Sorption
- Sorption of Calcium and Magnesium Ions
- Elution and Regeneration of Bio-Fix Beads
- Stability of BIO-FIX Beads
- BIO-FIX Bead Applications
- Continuous Column Test
- Passive System Technology
- Secondary Treatment of Waste Waters
- Summary and Conclusions
The removal of toxic metal contaminants from aqueous waste streams is one of the most important environmental issues facing the United States today. Although this issue has been addressed for many years, effective treatment options are limited. Chemical precipitation, ion exchange, reverse osmosis, and solvent extraction are the most commonly used procedures for removing metal ions from dilute aqueous streams. However, these procedures have significant disadvantages, such as incomplete metal removal, high reagent or energy requirements, and generation of toxic sludge or other waste products that require disposal. These disadvantages are particularly apparent at the low metal concentrations often encountered in waste waters.
The search for new and innovative treatment technologies has focused attention on the metal-binding capacities of biological materials. Peat moss, yeast, algae, bacteria, and various aquatic floras have been identified as organisms capable of sorbing toxic and heavy metals from dilute aqueous solutions. The mechanisms associated with metal sorption by biological materials are complex and involve both extracellular and intracellular metal binding. Extracellular metal accumulation has been reported as the more rapid mechanism and likely has the more significant role in metal sorption from waste waters.
Although living microbial populations are effective sorbents for toxic and heavy metals, available processing systems are cumbersome. Living biosorption systems often require the addition of nutrients, and maintenance of a healthy microbial population may be difficult due to the toxicity of the waste waters processed. Recovery of the metal-laden microorganisms from solution is troublesome due to liquid-solid separation problems.
Many species of yeast, algae, and other biomass sorb metals more effectively as a nonliving biomass, and their use eliminates the need to supply nutrients as well as the problem of toxic shock. However, recovery of the metal-laden biomass is still cumbersome. Researchers have recognized that immobilizing nonliving biomass in a granular or polymeric matrix may improve biomass performance and facilitate separation of biomass from solution. This approach is currently being pursued by the U.S. Bureau of Mines. Porous polysulfone beads containing nonliving biomass were fabricated and utilized to remove metal contaminants from acidic mine waters. Preliminary tests indicated that the beads, designated as BIO-FIX (an acronym for biomass-foam immobilized extractant), were readily prepared from commercially available raw materials, sorbed a variety of metal ions, and could be utilized in conventional hydrometallurgical processing equipment. Based on these initial results, additional studies were conducted to further define the bead fabrication procedure, bead sorption and elution characteristics, and bead stability. An additional objective of this investigation was to generate baseline data for pilot-scale field testing of BIO-FIX bead technology.
Fabrication of Bio-Fix Beads
Identification of Bead Fabrication Parameters
BIO-FIX beads were prepared by dissolving high-density polysulfone pellets in dimethylformamide (DMF), blending dried biomass into the solution, and injecting the slurry into water. Although durable beads were prepared using a wide range of fabrication parameters, studies were conducted to identify factors having the most significant effects on the chemical and physical characteristics of the beads. Variables investigated included the concentration of biomass in the beads, the polysulfone-to-solvent ratio used during bead preparation, the composition and temperature of the polymerizing media, and the bead curing time.
Only the biomass concentration and the polysulfone-to-solvent ratio had significant effects on bead performance. As expected, beads containing the greatest amounts of biomass extracted greater quantities of metal ions. However, metal sorption was not directly proportional to the biomass concentration. For example, beads containing 300 g of a blue-green algae (Spirulina sp.) per liter of beads were contacted for 24 h with a waste water containing 4.0 mg/L Mn and 0.06 mg/L Cd. Beads containing 150 g/L of the algae were also contacted with the waste water in a similar manner. The beads containing 300 g of biomass per liter extracted only 1.5 times as much manganese and 1.8 times as much cadmium as beads containing 150 g of biomass per liter. Surface area analysis indicated that the porosity of the BIO-FIX beads decreased as the biomass concentration increased. Subsequent microscopic examination revealed that many of the interior pores were tightly packed with biomass at the higher concentrations. Apparently, the dense biomass packing inhibited metal ion diffusion into the beads, and metal extraction efficiencies were not directly proportional to biomass concentrations. In addition, beads containing >300 g of biomass per liter were increasingly difficult to fabricate because of the increased viscosity of the polymer-biomass slurry. Thus, beads containing 250 to 300 g of biomass per liter were utilized throughout the test program.
The most significant effect of the polysulfone-to-solvent ratio was on the physical characteristics of the beads. Scanning electron microscope analysis indicated that the average pore size, and hence porosity of the beads, increased as the ratio of polysulfone decreased. The increased porosity enhanced the metal sorption kinetics. Nonlinear regression analysis indicated that a 40-pct increase in the rate of metal sorption was achieved when the polysulfone concentration used during bead preparation was decreased from 200 to 100 g/L of DMF. However, physical deterioration of beads prepared from solutions containing 75 g or less of polysulfone per liter of DMF was noted after several cycles. Also, problems were occasionally encountered during fabrication regarding sphericity and size uniformity with beads prepared from solutions containing 75 to 100 g polysulfone per liter of DMF. Thus, BIO-FIX beads prepared from solutions containing 100 g/L polysulfone exhibited a suitable combination of sorption kinetics and physical stability.
Selection of Biomass
The types of biomass studied in this investigation were selected following consultation with other researchers, literature searches, and sorption screening tests. Biological materials reported as having metal sorption capabilities were prepared by drying the material at 103° C for several hours. The biomass was then immobilized in polysulfone beads and contacted with waste waters containing arsenic, cadmium, copper, zinc, and manganese. The screening tests were conducted in a stirred reactor using an aqueous-to-bead (A:B) volume ratio of 20:1. Based on their sorption potential and availability, sphagnum peat moss, a marine algae (Ulva sp.), a blue-green algae, a yeast (Saccharomyces cerevisiae), common duckweed (Lemna sp), and alginate (a carbohydrate polymer) were selected for further study. Although each of these materials were effective metal extractants, sphagnum peat moss demonstrated the greatest metal sorption capacity from a wide variety of waste waters. In addition, peat moss is an abundant, low-cost material.
Standard Bead Fabrication Procedure
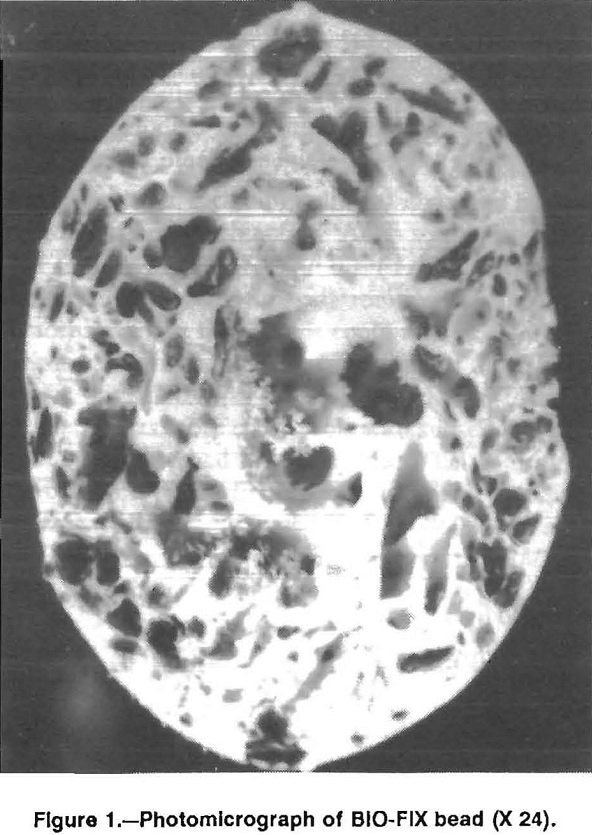
Based on results from the bead fabrication studies, a standard procedure was established for preparing BIO-FIX beads. Polysulfone (100 g/L of DMF) was moderately agitated at 25° C for 6 to 8 h in the solvent. Dried, minus 100-mesh biomass was blended into the polysulfone-DMF solution, and the resulting slurry was sprayed through an air atomization unit into water. Spherical beads formed immediately as the slurry droplets contacted the water. Bead size varied in response to the air pressure. Depending on the targeted application, beads ranging in size from 8 to 100 mesh were produced. The beads were moderately agitated in water for 16 h to allow excess DMF to leach out of the beads. The curing procedure also hardened the beads and ensured formation of a favorable pore structure. A cross-sectional photomicrograph (magnification of 24) of a cured bead showing the porous nature of the bead interior is shown in figure 1. The dark matter occupying several of the pores is immobilized algal biomass.
Bio-Fix Bead Sorption and Elution Characteristics
Several waste waters typical of those emanating from hard-rock mining districts were studied in this investigation. The waters included acid mine drainage waters and contaminated ground waters from copper, lead, gold, and zinc operations.
Metal Sorption from Dilute Waste Waters
Mining and mineral processing waste waters often contain concentrations of metal contaminants ranging from a few micrograms per liter to several milligrams per liter. Thus, a major task of this investigation was to determine the metal sorption characteristics of BIO-FIX beads at various concentrations.
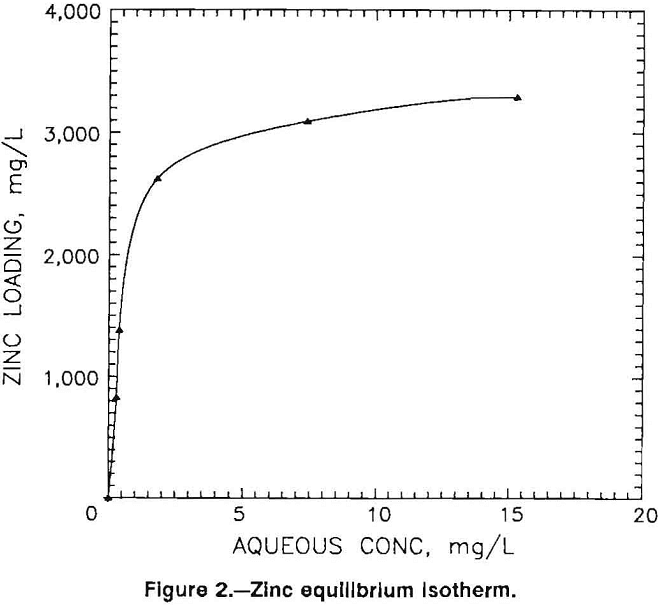
The effect of metal concentration on bead performance was evaluated by contacting sphagnum peat moss beads in a stirred tank with a pH 5.2 waste water containing 28 mg/L Zn. Minus 14- plus 24-mesh beads were used at A:B ratios of 30:1 to 250:1, and the contact time was 24 h. The loading isotherm generated from the data is shown in figure 2. The results illustrate that the beads exhibited significant zinc loadings over the entire concentration range. For example, even at an aqueous zinc concentration of only 0.5 mg/L, a loading of 1,600 mg Zn per liter of beads was achieved.
Further evidence of the sorption efficiency of BIO-FIX beads from dilute solutions was demonstrated by contacting waste waters containing arsenic, cadmium, copper,
and lead with peat moss or blue-green algae beads. These tests were conducted in fixed-bed columns and stirred tanks. Contact times were 10 to 20 min and the A:B ratio was 50:1 in each test. As shown in table 1, metal sorption was 97 pct or greater in each test, and all effluents met the federally mandated National Drinking Water Standards (NDWS).
Metal Sorption Kinetics
The rate of metal sorption by BIO-FIX beads was also investigated. Beads containing peat moss, blue-green algae, duckweed, or alginate were contacted in stirred tanks or fixed-bed columns with several waste waters. Test results with minus 14- plus 24-mesh beads indicated that >50 pct of the equilibrium extraction was generally obtained in the first 5 min of contact, while > 90 pct of the equilibrium extraction was achieved in 20 min. Data depicting cadmium extraction from a pH 3.8 water containing 0.071 mg/L Cd and zinc extraction from a pH 4.0 water containing 12.8 mg/L Zn are shown in figure 3.
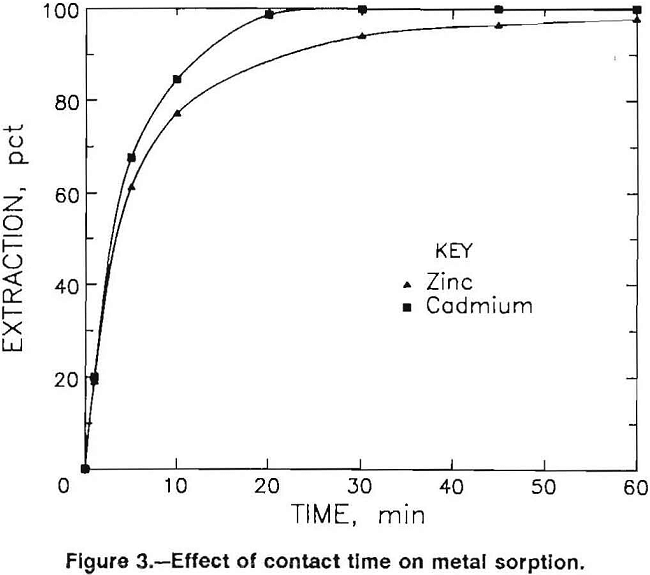
This pattern of metal sorption was evident throughout the testing program and was independent of the aqueous metal concentration, the biomass type, the A:B ratio employed, or the type of contactor utilized.
Effect of pH on Metal Sorption
Beads containing immobilized peat moss, algae, yeast, or alginate were contacted with several waste waters to determine the effect of pH on metal sorption. Initial tests yielded inconsistent results because of the variations in solution chemistry encountered in the waters. Subsequent tests were therefore conducted using a specific waste solution; the solution pH was varied by the addition of sulfuric acid (H2SO4) or sodium hydroxide (NaOH).
The most suitable pH range for metal sorption by BIO-FIX beads was 3 to 8. Equilibrium cadmium, copper, and other metal loadings at pH 2.0 were only 30 to 50 pct of those obtained from the same water at pH 4.0. Likewise, metal loadings at pH 9.0 were only 30 to 50 pct of those experienced at pH 7.0. However, exceptions were noted. Alginic acid beads removed 92 pct of the mercury from a pH 13.0 waste water containing 6.3 mg/L Hg, reducing the mercury level to 0.5 mg/L. Also, significant arsenic extractions were obtained from pH 1.5 to 2.5 waters using algae beads. These exceptions indicate that optimum utilization of biomass for a specific water may require correlation of the sorption characteristics of that particular biomass with the waste water solution chemistry.
Sorption of Calcium and Magnesium Ions
Many mining and mineral processing waste waters contain appreciable amounts of calcium and magnesium ions. Since many ion exchange resins readily sorb these ions, the capacity of the resins for toxic and heavy metals is decreased. The sorbed calcium and magnesium also interfere with elution and regeneration procedures. Unlike ion exchange resins, BIO-FIX beads exhibit selectivity for heavy metal ions over calcium and magnesium. This selectivity was demonstrated in a three-column fixed-bed circuit utilizing beads containing a blue-green algae. Fifty bed volumes (BV) of waste water from an inactive zinc mining operation was passed through lead and scavenger columns of the beads each cycle. As two columns were loaded, the third column (loaded during a prior cycle) was eluted. Elution of metal values was accomplished using 30 g/L H2SO4. In four loading-elution cycles, over 99 pct of the zinc, cadmium, and manganese were removed from the waste water. Calcium and magnesium ions were initially sorbed by the BIO-FIX beads, but were readily displaced from the loading columns as the beads preferentially loaded heavy metal ions. As a result, only 9 pct of the calcium and 4 pct of the magnesium were extracted and eventually reported to the acid eluate solution. This beneficial crowding effect was also observed in tests using peat moss, duckweed, or alginate BIO-FIX beads.
Elution and Regeneration of Bio-Fix Beads
Efficient removal of loaded metal values from BIO-FIX beads is necessary to ensure the long-term use of the beads for repeated extraction-elution cycles. Consultation with other researchers and a literature search indicated that dilute mineral acids, ethylenediamine-tetraacetic acid (EDTA), and caustic solutions have been utilized to remove sorbed metal ions from biological materials. Therefore, these reagents were evaluated for eluting sorbed metals from the beads studied in this investigation.
EDTA and caustic solutions were only partially successful in eluting metal contaminants from BIO-FIX beads. For example, solutions containing 0.1M to 0.3M EDTA stripped 75 to 85 pct of the zinc and 20 to 30 pct of the arsenic from marine algae or alginate biomass beads. EDTA also eluted about one-half of the copper and manganese from yeast biomass beads. Likewise, sodium carbonate (Na2CO3) and sodium hydroxide were effective eluates only in specific cases. For example, a 1M Na2CO3 solution removed >75 pct of the sorbed zinc from yeast and marine algae beads, but removed <30 pct of the manganese and arsenic from alginate biomass beads.
Mineral acids, including sulfuric, nitric, and hydrochloric, were effective eluants. Acid solutions containing 0.1M to 0.5M H2SO4, 0.5M to 0.1M HNO3 (nitric acid), or 0.1 M HCl (hydrochloric acid) removed essentially all of the sorbed metals from beads containing peat moss, yeast, duckweed, algae, or alginate biomass. Of the three acids, sulfuric was selected as the eluant of choice since the sulfate matrix was more compatible with subsequent processing of metal-enriched strip solutions. The majority of elution occurred in the first 15 min of contact, and elution was essentially complete within 1 h. Following acid elution, beads were rinsed for 20 min with a 0.1M Na2CO3 solution to neutralize any residual acid.
Stability of BIO-FIX Beads
The chemical and physical stability of BIO-FIX beads are important considerations in determining their operating life for waste water cleanup. Tests were therefore conducted to determine bead performance over repeated extraction-elution cycles. Beads containing sphagnum peat moss were contacted with a pH 3.8 mine drainage water containing 11.6 mg/L Zn, 6.5 mg/L Mn, and 0.065 mg/L Cd in a fluidized-bed column. Fifty bed volumes of waste water was processed each loading cycle, and elution was accomplished using 20 g/L H2SO4. After 100 extraction- elution cycles, the beads showed no decrease in sorption or elution efficiency, and no physical deterioration of the beads was observed. Metal extraction efficiencies remained constant throughout the 100 cycles and averaged >99 pct for zinc, manganese, and cadmium. In shorter term tests, excellent stability was maintained during 75 cycles with beads containing immobilized duckweed, and 44 cycles with beads containing alginate. Extraction trends and visual observations indicated that stability of the beads for additional cycles was likely.
The excellent physical stability of BIO-FIX beads was also demonstrated by placing a 5-ft bed of beads in an ion- exchange column. Water was continually pumped downward through the beads for 30 days at a flow rate of 10 BV per hour. Physical analysis of the minus 14- plus 24-mesh beads after 30 days indicated that degradation due to attrition was nil.
Further evidence of BIO-FIX bead stability was demonstrated by exposing beads containing peat moss to sunlight and varied climatic situations, such as freeze-thaw and wet-dry cycles. After 75 days of exposure, none of the beads showed signs of physical deterioration. In addition, bead extraction and elution characteristics were identical to those of a control set.
BIO-FIX Bead Applications
After determining the bead sorption and elution characteristics, several circuits were assembled and operated to further define potential applications for this technology. Generation of data applicable to scale-up and field testing was also initiated.
Continuous Column Test
Continuous treatment of an acidic mine water emanating from an inactive zinc mining operation was demonstrated in a three-column circuit containing peat moss BIO-FIX beads. The pH 3.8 water contained 11.7 mg/L Zn, 6.0 mg/L Mn, and 0.058 mg/L Cd. To satisfy all discharge requirements, a treated effluent containing <0.047 mg/L Zn, 0.05 mg/L Mn, and 0.01 mg/L Cd was required. Eighty bed volumes of water was passed through lead and scavenger load columns of the beads each cycle at a flow rate of 30 BV per hour. Elution was accomplished using 20 g/L H2SO4.
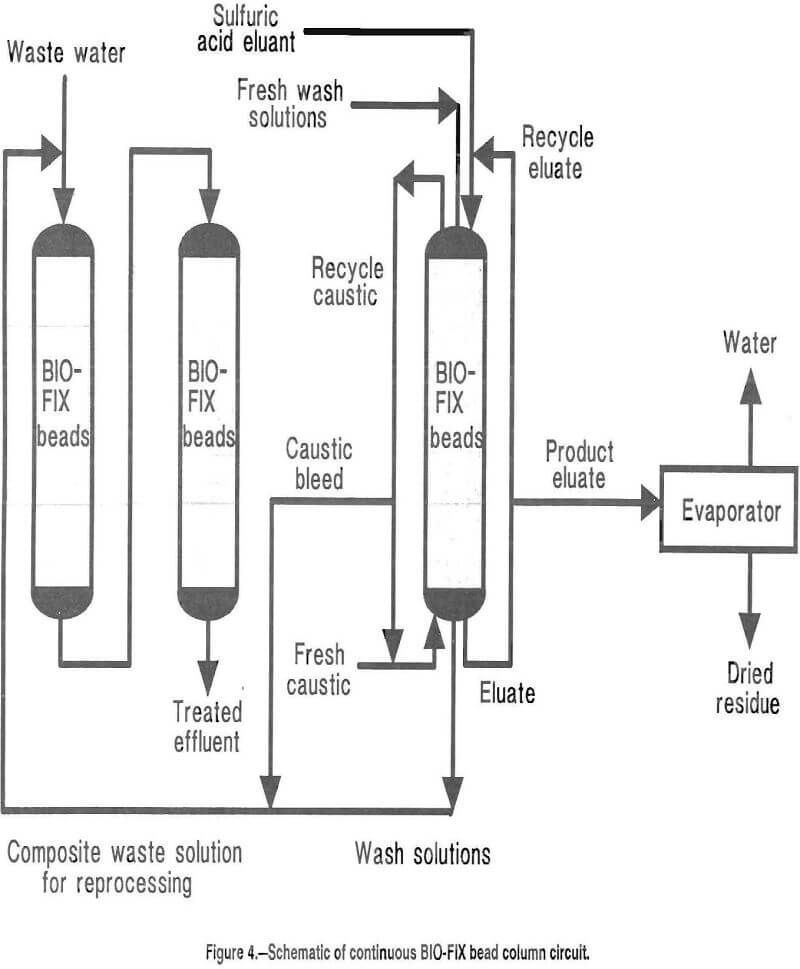
Several hundred gallons of the waste water was processed in cyclic tests. The beads extracted >99 pct of each metal contaminant, and discharge criteria were consistently met. The majority of the acid strip solution was recycled to each successive elution and was progressively enriched. The small amount of product eluate removed from the circuit each cycle contained about 200 times as much zinc, manganese, and cadmium as the original mine water. The product eluate was evaporated to dryness, and the resulting residue contained 9.5 pct Zn, 5.0 pct Mn, and 0.06 pct Cd. Initial contacts with an industrial zinc processor indicate that this residue is suitable for further processing and subsequent recovery of metal values. In addition, all wash and regeneration solutions were recycled within the circuit, and thus, no hazardous wastes requiring disposal were generated. A schematic depicting treatment of this waste water is shown in figure 4.
Passive System Technology
Because of their excellent metal sorption properties and long-term stability, the use of BIO-FIX beads in passive treatment circuits was investigated. Removal of metal contaminants from waste waters using circuits with low maintenance and labor requirements would avoid the high operating expenses associated with active treatment procedures such as chemical precipitation.
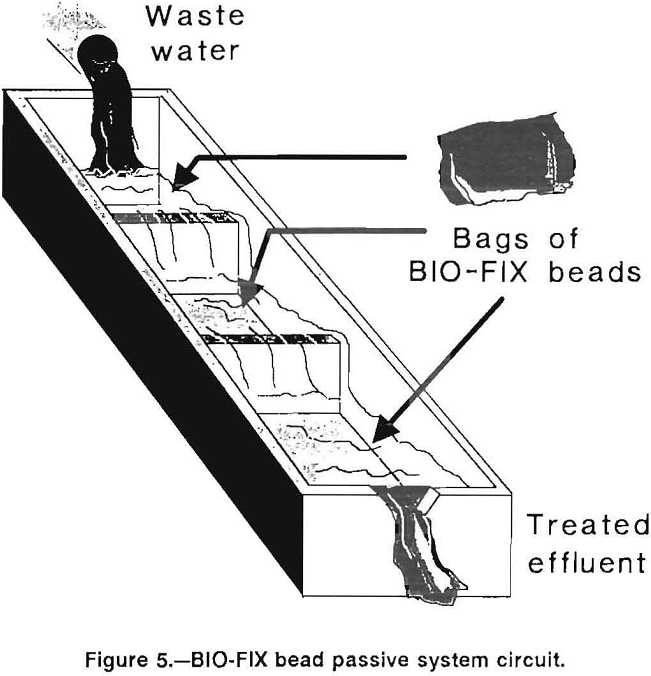
A promising system utilizing BIO-FIX beads consists of enclosing the beads in porous bags, placing the bags in a trough or lined trench, and allowing waste water to flow through the bags. A compartmented trough designed to utilize the natural hydrostatic head of a waste stream was constructed. Waste water flowed by gravity through a series of bags, and metal ions were progressively removed by the beads. As the bags of beads near the head of the trough became fully loaded with metal ions, they were removed and regenerated. Meanwhile, bags at the bottom
of the trough were moved to the front and replaced with freshly regenerated bags. Thus, the flow of waste water was countercurrent to the movement of the bags of BIO-FIX beads, providing an efficient system for removing all traces of metals from the waste water. In a field situation, bag replacement frequency will be site specific and will be a function of waste water flow rate, metal contaminant concentration, and pH. Figure 5 presents a conceptual illustration of this system.
An important consideration in utilizing BIO-FIX beads in porous bags was selection of a suitable bag material. Several potential materials were evaluated to determine physical strength, chemical resistance to acid and caustic solutions, and wetting characteristics. Polymax B, a fine-mesh filter media woven from polypropylene fibers, met all the above criteria. Bags fabricated from Polymax filter media were resistant to tearing, and no decrease in strength was noted after immersion in 1M H2SO4 or 1M Na2CO3 for 2 weeks. Bag wetting characteristics were determined by enclosing peat moss beads (minus 10- plus 14-mesh) in Polymax filter media bags and contacting the bags with a zinc-manganese waste water. In 10 min, the beads extracted 93 pct of the zinc and 89 pct of the manganese. Extractions after 20 mm were 96 pct Zn and 94 pct Mn. The rate of metal extraction was identical to that obtained using peat moss beads contacted directly with the waste water, indicating that excellent solution-to-bead contact was achieved in the bags.
Secondary Treatment of Waste Waters
As discussed in the “Introduction” section, chemical precipitation is one of the most widely used processes for removing metal contaminants from aqueous wastes. However, effluent concentrations that meet the NDWS are sometimes difficult to achieve using precipitation alone, and secondary treatment is necessary. Since BIO-FIX beads readily sorb metals from the near-neutral solutions resulting from precipitation processes, use of the beads as a secondary or “polishing” technique was investigated.
A complex waste water was subjected to chemical precipitation by adding a slurry of calcium carbonate (CaCO3) until a final pH of 7.0 was reached. The pH was maintained for 4 h, at which time the solution was filtered and sampled. The filtrate contained, in milligrams per liter, 1.3 Ag, 0.08 Mn, and 0.01 Pb. BIO-FIX beads at an A:B ratio of 30:1 containing marine algae were then contacted with the filtrate in a stirred tank. After 1 h of contact, 90 pct or greater of each targeted contaminant was re moved from the filtrate, and the NDWS were met. The resulting metal ion concentrations, in milligrams per liter, were 0.011 Ag, 0.002 Mn, and 0.001 Pb. In comparative tests, a pH approaching 10 was required before precipitation alone achieved these levels. However, nearly twice as much reagent was consumed to reach the higher pH,
and the volume of sludge generated was about twice as great at pH 10 as that generated at pH 7.
Summary and Conclusions
Porous polysulfone beads containing immobilized biological materials removed arsenic, cadmium, copper, lead, manganese, and zinc from several waste waters. These toxic and heavy metals are among those most commonly encountered in acid mine drainage waters. Metal concentrations in the waste waters were generally reduced to <0.1 mg/L, and treated effluents frequently met NDWS. The beads were fabricated from readily available raw materials and demonstrated excellent handling characteristics in stirred tanks, fixed-bed columns, fluidized-bed columns, and a low-maintenance passive system consisting of beads enclosed in fine-mesh bags.
Sorption of metal contaminants by BIO-FIX beads was rapid. Greater than 50 pct of the equilibrium sorption was obtained in 5 min, while >90 pct sorption was achieved in 20 min. Elution and regeneration of the beads were accomplished using dilute mineral acids and caustic solutions. Recycling of acid strip solutions yielded product eluates that were amenable to further processing using conventional hydrometallurgical techniques. No decrease in sorption or elution efficiency was observed when BIO-FIX beads were cycled continuously for up to 100 extraction-elution cycles.

