Copper Agitated Leaching and cementation process of copper problem: The treatment of copper ores, both oxides and sulfides by means of flotation, hydrometallurgy or other processes has been well established for many years. Flotation has been applied generally to the treatment of sulfide ores, both in small and large scale operations.
Hydrometallurgical methods alone or in combination with flotation have mainly been applied to large tonnage operations to recover copper from low grade native copper, oxidized, or mixed oxide and sulfide ores that are not readily concentrated by flotation alone or by other means, either due to treatment costs or low recovery. The treatment of smaller tonnages of such ores by hydrometallurgy has not been widely employed due to high capital plant costs.
CHEMISTRY OF COPPER DISSOLUTION
The dissolution of copper minerals is basically simple decomposition in the case of oxidized minerals, and oxidization-reduction for sulfide minerals. The resultant products are water soluble. The reactions of the common copper minerals with sulfuric acid and ferric sulfate are briefly outlined below.
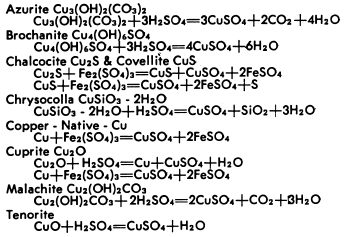
The dissolution of the various oxides, carbonates, silicates and sulfides occur at different rates which can greatly affect recovery. Each ore offers its own problems and the associated gangue may undergo reactions to form soluble salts to result in excessive consumption of solvent or to produce an adverse effect on copper bearing solutions. These conditions can only be determined by thorough and adequate laboratory and pilot plant test work to establish all factors essential to process development.
The Copper Leaching and Copper Cementation Process
The method of dissolution by sulfuric acid and ferric sulfate with chemical precipitation by means of scrap or sponge iron appears to offer the best potential for wide application and low costs. Its basic chemistry is not complex but its economic application is dependent on handling of materials and solutions by such procedures to insure low operating costs.
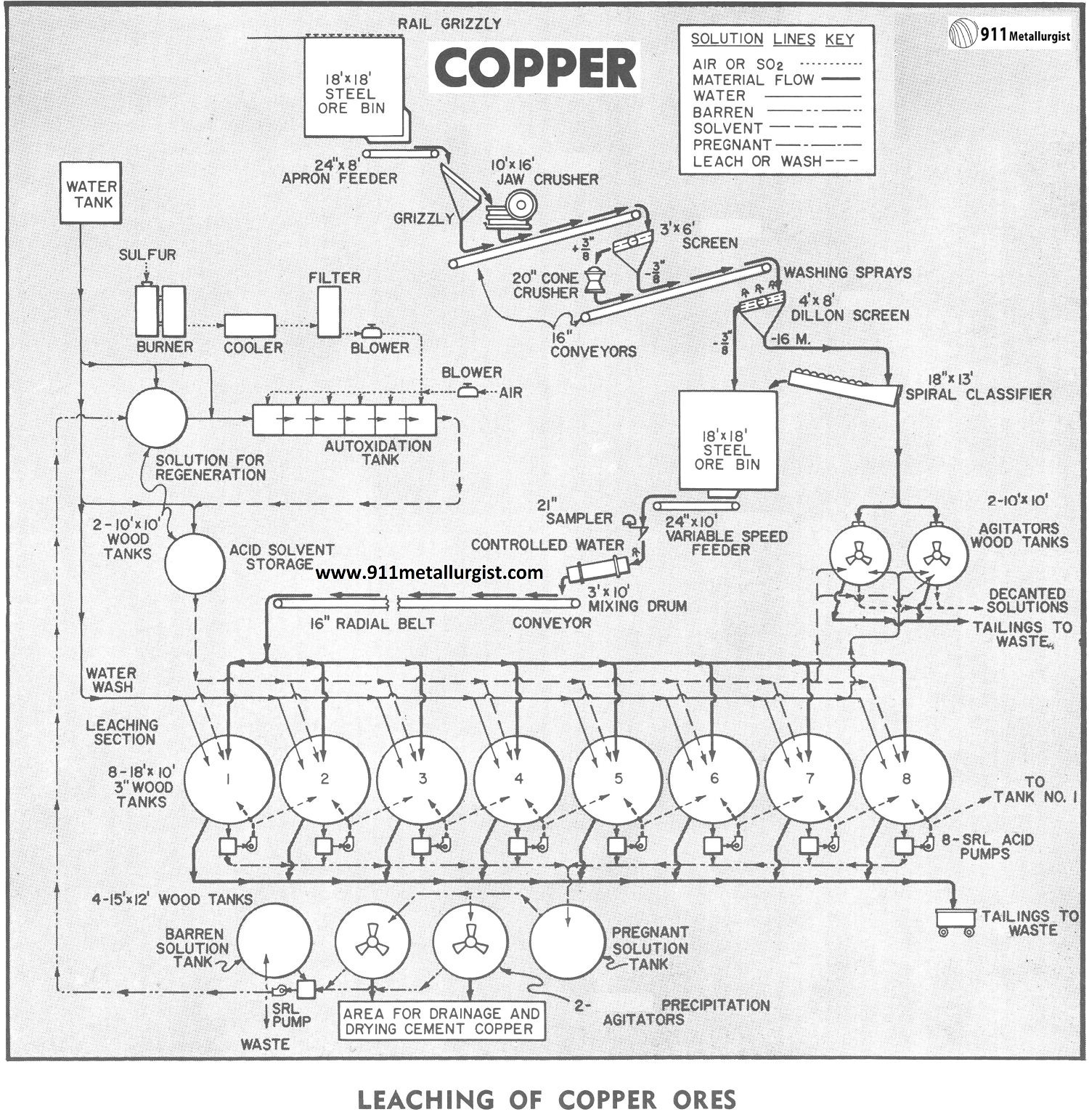
The flowsheet discussed in this study is for treatment of 100 tons per day oxide-sulfide copper ore by the leaching process using sulfuric acid-ferric sulfate solvent. This flowsheet would also be suitable for a larger tonnage by proper sizing of the various units of equipment. The flowsheet contemplates a six to seven day leach-wash cycle with one tank being discharged and charged each day.
In leaching plants of capacity illustrated by this flowsheet recovery of copper normally effected range from 80 to 90% of oxide copper and 70% or more of sulfide copper from average ores.
PREPARATION OF THE COPPER ORE
This study is limited in general to percolation leaching as it is the most widely used method to give proper contact of solvent with the ore to effect extraction of the copper.
One of the important factors in percolation leaching is the size reduction necessary for copper recovery within reasonable time limits.
It is self evident that the larger the size of particle that can be readily leached, the less the cost of crushing becomes and the smaller the particle the less time required for extraction. Therefore a size must be selected to give a minimum of fines, and with greatest uniformity as may be possible that will permit leaching within economic time limits. In most cases crushing to minus 3/8″ has been satisfactory.
If the crushed ore contains excess fines it may be advantageous to add a coarse fraction of ore or waste material to avoid the necessity of removing fines.
STORAGE AND FEEDING THE ORE
Steel ore bins of field welded tank type are used since they can be secured in most areas and installed at a lower cost than other forms.
The minus 3/8″ ore from fine ore storage is controlled by a variable speed feeder to a cylindrical mixing drum for aglomeration with water before being conveyed to charge leach tanks. The conveyor is mounted to radial movement for charging any of the tanks which are also arranged to a conforming radius for ease of loading by use of a single conveyor.
The fines produced in crushing or slimes contained in the ore interfere with leaching and lowers extraction due to channeling of solution through the coarser segregated particles. Channeling can be reduced by methods to insure uniform mixing and charging of leach tanks. Excess fines often must be removed and treated separately by agitation. This however is an added complication in a small plant by adding to initial plant costs with extra labor and attention being required to handle.
In those cases where fines must be removed, due to channeling or poor porosity, all or a portion can be separated by wet or dry screening followed by classification to remove slime fraction for treatment by agitation and decantation methods, as illustrated in flowsheet.
In some cases agglomeration of fines has been effective to eliminate the need for separate treatment of fines. This is accomplished by adding water or solution to the crushed ore to bring the moisture content up to about 10% and mixing in a cylindrical mill or pug mill. By mixing, rounded particles are formed in which the fines adhere to the coarser particles to give a more uniform mass with improved permeability.
COPPER LEACHING
The application of hydrometallurgical leaching to oxide and combined oxide and sulfide ores depends mainly on obtaining solvent at low cost and in holding initial plant costs within feasible limits. Dilute sulfuric acid-ferric sulfate has been found to be an excellent solvent for oxide minerals as well as for such sulfide forms as chalcocite and covelite but not chalcopyrite.
Percolation can be either upward with solutions entering at the bottom and overflowing at the top or downward where solutions are added at the top with bottom drainage.
CHARGING THE AGITATED LEACH TANKS
In charging tanks care must be taken to spread the charge evenly to avoid segregation as much as possible to reduce channeling of solution. If the ore has been agglomerated less care is required in charging since segregation is greatly reduced. Drainage of tanks is often improved by first placing a layer of coarse ore several inches in thickness over the filter bottom before charging.
Also, when downward percolation is used, a similar layer of coarse ore spread over the top will aid in flow of solutions uniformly over entire tank area and help break the impact of solutions from distribution pipes.
Several methods of percolation leaching can be employed. One is where solutions fully cover the entire charge for a certain soaking period followed by drainage and successive treatments with solutions of decreasing acid strength, each being followed by a drainage period. During each cycle the solution can be circulated by pumping to give a constant flow through the charge.
Another method of considerable merit is open drainage leaching where the solution is added by spray or other distribution means at a rate below the rate of percolation so that the charge does not become flooded. By proper distribution the solution covers the ore particles with a film of solution, leaving air spaces between which aids in the dissolution of any native copper or cuprite. Tests have indicated up to 50% increase in recovery of copper from cuprite due to excess oxygen being available by this method.
Counter current leaching is used in this discussion with the individual charges in each tank being treated successively with higher concentration of solution. The greatest strength solution being applied to a charge for its final cycle of predetermined soaking and/or recirculation before being drained and advanced to the next tank. The process proceeds from tank to tank with a continual decrease in acid strength and increase in copper concentration until it is finally withdrawn as pregnant solution from the charge which has had its first leach cycle. A weak acid wash is often added to this charge from which the pregnant solution has been drained to remove as much of the copper-rich occluded solution as possible. This wash joins the pregnant solution ready for precipitation.
After the final leach cycle for a charge, it undergoes one or more wash cycles with water to remove any remaining copper in solution. In some cases this represents as much as 30% of total copper dissolved and effective washing is of major importance. Part of such washes may be added to pregnant solution with balance advancing counter-currently.
COPPER PRECIPITATION & CEMENTATION
The method for recovery of copper from leach solutions by precipitation with iron has been known for many years, being reported in use by Rio Tinto Mines in Spain as early as 1752. Scrap iron, detinned cans and sponge iron have been used depending on cost and availability. Labor costs involved in the use of scrap iron and cans are usually higher than with the use of sponge iron.
The basic reaction for precipitation is simple, however in practice other reactions may occur to affect the amount of iron consumed and the grade of cement copper produced. The amounts of free acid and ferric iron in solution affects the amount of iron used and if the solution is near neutral little or no copper will precipitate.
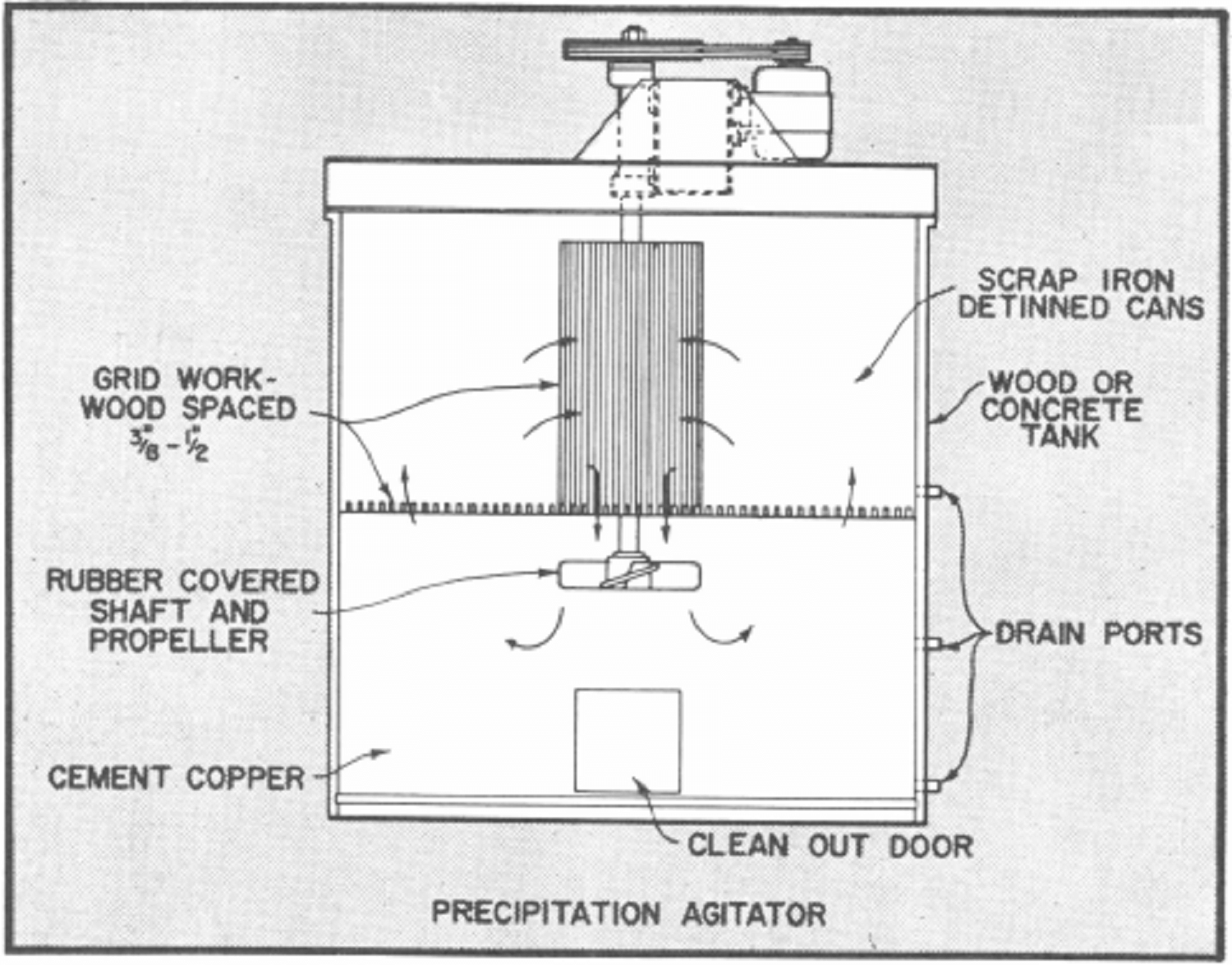
Bottom side doors are provided in the agitator tanks to allow the cement copper to be removed by hand methods for further drainage and drying. The cement copper is normally sacked for shipment.
Theoretically one pound or iron will precipitate 1.137 pounds of copper while in actual practice two to three times this amount is required especially if the iron used is highly oxidized.
Sponge iron is usually more effective and consumption is often equal to weight of copper precipitated. Impurities in the iron lower the grade of cement copper produced. The grade of cement copper normally ranges between 50 to 90% copper.
Recovery of over 97% of the copper in solution is not unusual from solutions containing copper in the range of 1.0 to 15.0 GPL.
As noted in the flowsheet, the pregnant solution from storage is transferred on a batch basis to one of two precipitation agitators to which iron in the form of light scrap or detinned cans has been added to supporting grid.
After filling an agitator with pregnant solution it is placed in operation to keep the solution in movement through the iron to effect precipitation of the copper as rapidly as possible. Usually 2 to 3 hours agitation is adequate to complete the reaction depending upon acid strength and other factors.
The barren solution is held in the agitator until the copper cement settles and is then transferred to the barren solution tank. The copper cement can be removed at the end of each cycle or after several cycles of operation.
ACID SUPPLY
The source of sulphuric acid for leaching is usually a major consideration for small plants. If the plant is located in an area where acid can be purchased at low cost, one of the greater items of capital expense can be avoided. For most plants the acid must be produced.
PRODUCTION OF SULPHURIC ACID
Since an acid strength of above 7% is not normally required, the “Autoxidation” process for acid production can be set up for less expense than for other processes. The autoxidation method has been successfully used for some years in small plants in Europe, South America and in the United States. In this process ferrous sulfate is used as a catalyst at a rate of about 5% of the acid produced and makes sulfuric acid-ferric sulfate solvent through autoxidation of ferrous sulfate and sulfur dioxide in the presence of excess air.
The ferrous sulfate contained in the barren solution after copper precipitation, diluted with water to desired iron content is fed to the autoxidation cell together with sulfur dioxide with excess air to produce the sulfuric acid-ferrous sulfate solvent.
The sulfur dioxide required is produced by the burning of elemental sulfur. The sulfur burner, with a capacity for burning about 2000 pounds per day, consists of a brick lined steel cylinder containing a sulfur melting pot above sulfur burning trays. Sulfur is fed through the top of the burner and on melting flows downward to fill burning trays. The burning rate is controlled by regulation of air inlet rate with the gas produced containing 6 to 8% sulfur dioxide. A combustion chamber, cooler and filter follows the burner with the gas being compressed by a stainless steel positive blower for admission to the autoxidation cells together with air supplied through a separate blower.
The reaction first oxidizes the ferrous sulfate, to ferric sulfate and then produces sulfuric acid. Under proper control about 90% of the ferrous iron is converted before the reaction makes sulfuric acid. The acid strength can be built up to or above that normally required for leaching.
The main requirement for the reaction is to keep the solution in contact with the gases in a manner that both the SO2 and oxygen will be absorbed. SO2 gas is more soluble in water than is oxygen and it is necessary to admit the gas into the solution in fine bubbles, for effective results. Capacity per square foot of cell area using 6 to 8% SO2 gas ranges above 75 pounds of 100% sulfuric acid or equivalent in ferric sulfate per 24 hours, however due to variables the average capacity may be about 50% of this amount. Efficiency of SO2 gas fed to cells is normally in the order of 97%. For best operation the solution fed to cells should contain between 0.5 to 0.7% total iron, however if ferric sulfate requirements are above this then greater amounts of iron can be returned to the cells for regeneration.
The cells illustrated are of reinforced concrete construction with lead lining, equipped with aluminum oxide porous media for diffusion of SO2 gas and air into the solution. Porous aluminum oxide tubes are also used to supply both gas and air into similar cells. Pressures for admission of gases ordinarily are between 3 to 4 PS1.
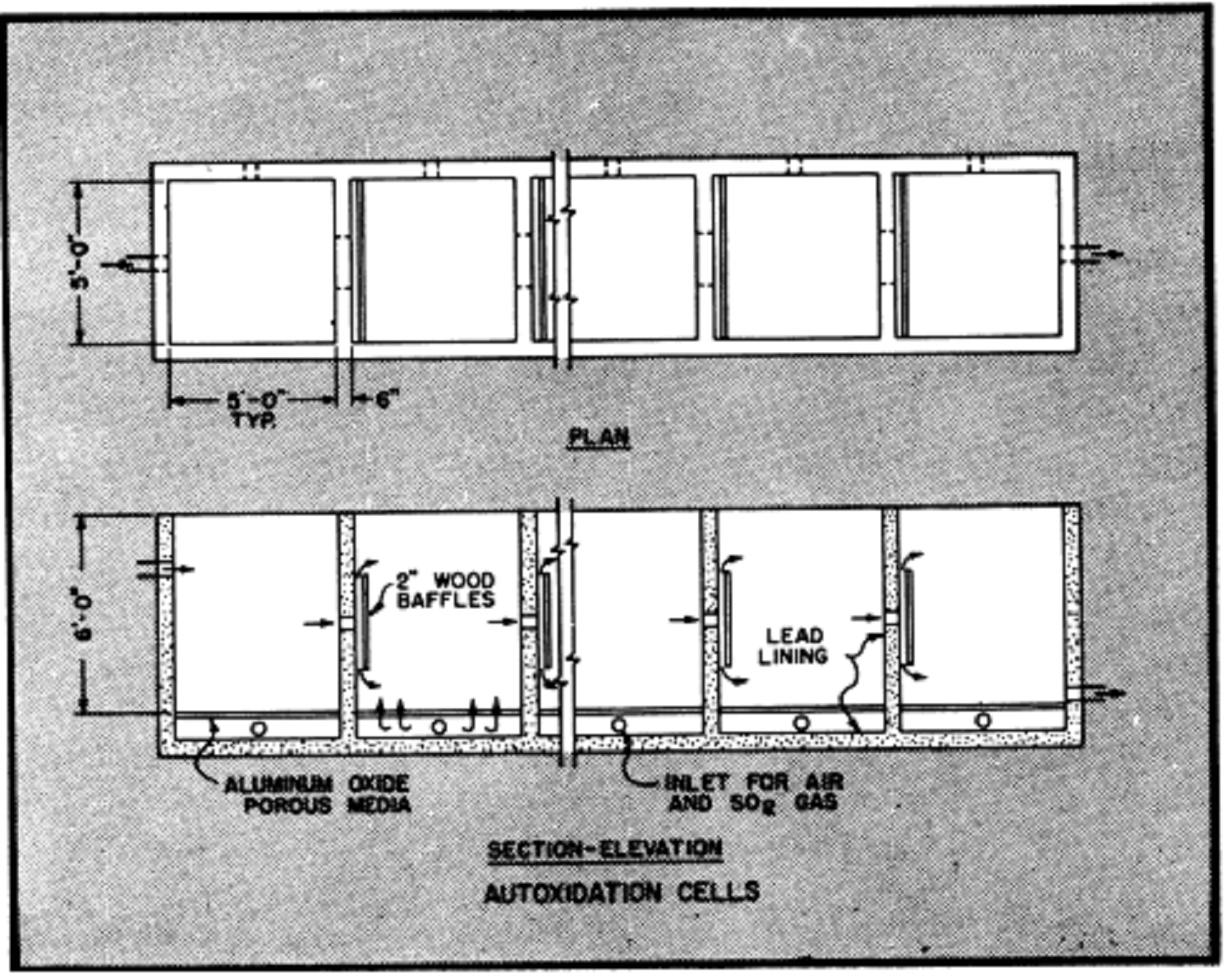
The autoxidation cells shown have an area of 150 square feet and can produce in excess of 6000 pounds of 100% sulfuric acid or its equivalent in ferric sulfate per day. The flow rate through cells is controlled at a rate to produce solvent with an acid strength of 3 to 7% sulfuric acid as may be desired. Approximately five percent of the barren solution is returned to the cells for regeneration and to provide the ferrous sulfate necessary as a catalyst. Additional amounts can be returned for regeneration of ferric sulfate if solvent requirements for dissolution of sulfide copper makes it essential. The balance of the barren solution is discarded to waste.
Bulletin No. 321 of U. S. Bureau of Mines gives data for calculations in regards to performance of autoxidation cells.
MATERIALS OF CONSTRUCTION
Materials of construction are limited due to the corrosive nature of the solution containing free sulfuric acid, ferrous and ferric sulfate, copper sulfate, and other possible salts and acids. These in combination are more corrosive than any one singly. Materials are usually limited to lead, Duriron, Hastelloy B, C and D, rubber, bitumastic cements, asphalt, porcelain, vitrified clay products, glass and certain plastics such as P.V.C. Concrete made with additives to improve density is widely used for tanks which are coated inside with bitumastic, asphalt or laminated plastic and fiber glass.
MATERIAL HANDLING
Material handling is an important economic factor and the methods used may depend largely on the nature and conditions at the plant site, labor rates and capital requirements.
Leach tanks can be located radially in order to use one conveyor, pivoted at its loading point and arranged to be moved on an arc to fill any tank.
Uniform distribution of materials in filling tanks can be done either by hand, mechanical distribution or shuttle conveyors.
Methods used in discharging tanks vary from shoveling all material over tank sides into cars or onto conveyors to discharge by shoveling through bottom or side doors to the use of scrapers or clam shell excavators. Also where adequate water is available the leached material can be flushed by jet streams through bottom doors into launders for disposal. This requires adequate tailings area with proper gradient for flow of coarse material to disposal area.
A new look at Agitated Tank Copper Leaching
Agitated Leaching
The agitated leaching method is limited to those ores, or to that portion of those ores, that because of particle size do not permit free passage of leach solution through the interstices between the ore particles and. thus, cannot be effectively treated by heap or vat leaching. In this process, dissolution is effected by keeping the finely divided ore particles in suspension in the solvent. This is usually carried out in an agitated vessel, and the solids are dispersed in the liquid either by gas injection or by a rotating impeller. Dissolution of the ore may be either on a batch basis (a given charge of ore is agitated with required amount of leach solution until achieving optimum extraction) or on a continuous basis (ore and leach solution are added simultaneously. However, instead of dissolution in a single vessel, the ore-solution mixture passes from vessel to vessel in series until achieving optimum extraction. Pachuca vessels, with high lifts, are convenient for this type of agitation. A pachuca is a cylindrical tank with a conical bottom and contains a pipe that is coaxial with the leaching tank and is open at both ends. Compressed air is introduced, at the lower end of this pipe, which behaves as an air lift. The density of the pulp within the pipe is less than that of the pulp surrounding it, because of the column of air bubbles contained in the pipe; the pressure of the denser pulp causes the pulp in the central pipe to rise and overflow, thus circulating the entire charge. The pachuca vessel is extremely simple in design and has no moving parts. Essentially the only difference in the batch and continuous leach is in the feed and discharge mechanics, i.e., a central system vs. a number of individual circuits. In either method variations can be made in how. when, and where additions of either ore or leach solution are made. When the agitated leach method is used for pressure leaching operations, the leaching is carried out in an autoclave.
Two major differences are evident between agitated and percolation leaching methods. First, in agitated leaching, the liquid is the continuous phase, and second, in this form of leaching turbulent flow conditions exist, whereas in percolation the flow is more usually laminar. There is, therefore, a considerable difference between the rates of mass transfer obtained in the two types. The higher transfer rates obtained under turbulent contact are achieved by a higher power input to sustain the turbulence.
In the context of processing of low-grade copper ores, particularly oxide-sulfide mixed ores, which arc difficult to concentrate via flotation or other techniques, a leaching process named thin layer leaching, originally proposed by Holmes and Narver, Inc.29 has attracted attention. The process involves two principal steps:
- a curing step in which the finely crushed moistened ore is attacked with strong sulfuric acid; the application of acid is followed by a curing or aging period;
- the second step involves leaching with diluted sulfuric acid in a manner of normal heap leaching. This technology results in higher leaching rates than in normal vat leaching and a reduced acid consumption.
