The photograph, Fig. 1, shows the apparatus a little less than half size, consisting of a filtering-flask fitted with rubber stopper through which passes a bent glass tube, and an extraction-thimble fitted with rubber stopper through which passes a glass tube of 0.25-inch bore. Both tubes are connected by a short piece of rubber tubing.
A section of a thimble is shown in the photograph; the tube extends to within 1/8 in. of the tapered end.
The object of using the thimble is to remove the acid from the beaker after all the copper has been precipitated. Time is saved, the copper is not exposed to the acid alone, and there are none of the losses attending ordinary filtration. I have accomplished these results by means of a piece of perforated platinum fastened in the end of a 0.25-in. bore glass tube and a filter-mat of asbestos, but after my supply of proper length fiber became exhausted I could not replenish it even after purchasing 14 lots from four different dealers.
The apparatus may be used to remove at least seven-eighths of a supernatant liquid from a settled precipitate without disturbing the latter.
The application of the thimble is best shown by partly outlining the assay for copper, as follows:
Dilute the acid solution of copper and other sulphates to 150 cc. in a 200-cc. Jena beaker, place on a hot plate, add 2 drops of concentrated HCl, then place a strip of aluminum in the beaker (this may be bent or straight, as desired). Connect the apparatus, as shown in Fig. 1, with a filter-pump having a strong suction. When the copper is precipitated, remove the beaker from the hot plate and insert the extraction-thimble alongside the strip of aluminum. The acid solution will be drawn-through the porous tube. Wash the upper end of the aluminum strip with a jet of hot water, wash down the sides of the beaker, and add about 25 cc. of hot water to cover the precipitate.
As soon as all the liquid is out of the beaker, disconnect the thimble by slipping the rubber tubing from the bent glass tube of the filtering-flask, wash it with a jet of hot water. If there is no adhering copper, lift from the beaker, add 5 cc. of strong nitric acid to the precipitated copper, and carry out the titration in the usual manner. If the copper is in a finely-divided condition as a precipitate on the aluminum, it will adhere as a black coating on the thimble, but as soon as the thimble is disconnected the water inside it will ooze out through the pores, and if the 5 cc. of strong nitric acid be poured on it, the copper will immediately dissolve and the thimble may be washed with a jet of hot water. The filtration is more rapid when the solution is hot.
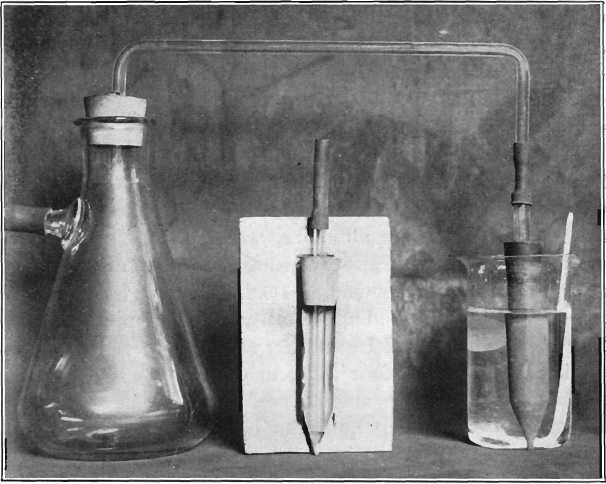
Fig.-1 The Alundum Extraction-Thimble as Used in the Determination of Copper.
If the last of the solution in the beaker is not withdrawn as rapidly as the first, tip the beaker at an angle of 45°. With a pump having a strong suction this will not be necessary. The thimble attached to the flask, as shown above, was used in more than 300 determinations.
The Alundum Extraction-Thimble Used in the Determination of Copper. BY L. W. BAHNEY, NEW HAVEN, CONN.
Standard Methods of Chemical Analysis: A Manual of Analytical Methods and General Reference
