Ores containing silver in Arsenic and Antimony combinations offer considerable difficulties in cyanidation and it has usually been considered that the removal of these compounds by concentration was the only way to deal with them. G. H. Clevenger, however, when working on the ores of the Nipissing Mining Company of Ontario, Canada, found that by grinding exceedingly fine and treating with strong solution for four or five days a high extraction could be obtained. The silver occurred partly as sulphide, sulphantimonide and sulpharsenide and partly as metallic silver and dyscrasite, an alloy of silver and antimony in varying proportions.
To accelerate the dissolution of the antimony and arsenic compounds J. J. Denny introduced a process that had for its object their decomposition in a preliminary treatment. This process consists in grinding the whole of the ore to a slime and subjecting it to the action of metallic aluminium in a caustic soda solution, after which it is filtered and subjected to cyanidation.
By the preliminary treatment the silver, and in part at least, the antimony and arsenic, are reduced to the metallic state, and are so found. The reduction is accomplished by the nascent hydrogen resulting from the action of caustic soda on the aluminium according to the following equation:
(1) 2Al + 2NaOH + 2H2O = Na2Al2O4 + 6H.”
The probable reactions involved in complete reduction are indicated by the following equations:
(2) Argentite.
6H + 3Ag2S + 6NaOH = 3Na2S + 6H2O + 6Ag
(3) Pyrargyrite.
6H + Ag3SbS3 + 6NaOH = 3Na2S + 6H2O + 3Ag + Sb
(4) Proustite.
6H + Ag3AsS3 + 6NaOH = 3Na2S + 6H20 + 3Ag + As.”
“The reactions being reversible, probably the arsenic and antimony are not completely reduced to the metallic state in practice, and the investigation of the subject is rendered difficult by reason of secondary reactions by which the arsenic and antimony are possibly redissolved to form arsenates and antimonates by the excess caustic of the reducing solution, and the protective alkali of the cyaniding solution. The working solution shows the presence of these compounds, but in practice they are found to have no detrimental effect either in the reducing or the cyaniding treatments. The solution assays, antimony 0.0084% and arsenic 0.026 percent.”
In practice the difficulty has been to obtain adequate contact between the refractory mineral particles and the aluminium surface. Various expedients have been tried but so far the best results have been obtained by first passing the pulp through a tube mill loaded with small aluminium ingots instead of pebbles, and then sending it to an agitator tank lined with aluminium plates, in which it is kept agitating for about 12 hours. Even so the action is incomplete because if a sample of the pulp be taken after treatment is finished and divided into two parts, one of which is cyanided direct and the other subjected to further desulphurizing in a bottle and then cyanided, the latter will invariably yield a better silver extraction.
The dyscrasite is unaffected by this treatment, but will dissolve in the cyanide solution, given time and sufficiently fine comminution.
As regards costs the above mentioned article gives the following figures:
“In connection with the desulphurizing treatment, treating 7268 tons per month, the following data are available:
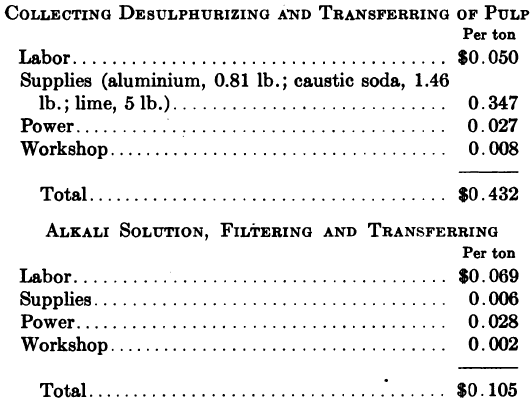
“The desulphurizing treatment effects a saving of from one to four ounces per ton, depending on the amount of refractory minerals present, at a total cost of 54 c. per ton.”
In the cyanidation of this ore, whether previously desulphurized or not, it was found that a serious deterioration occurred in the extractive power of the stock solution after repeated precipitation by zinc, an effect which was shown to be due to some obscure interaction between the zinc gradually accumulated in the solution and the arsenic and antimony derived from the ore. This was overcome by the substitution of aluminium for zinc as a precipitant, a method which proved so efficacious that the stock solution after the plant had been running twelve months was more active in dissolving power than a freshly prepared cyanide solution.
