Copper occurs native in large quantities, especially in the Lake Superior district ; in this state it is generally pure. More frequently it is found in combination. The ores of copper may be classed as oxides and sulphides. The most abundant oxidised ores are the carbonates, malachite and chessylite; the silicates, as also the red and black oxides, occur less abundantly. All these yield their copper in solution on boiling with hydrochloric acid.
The sulphides are more abundant. Copper pyrites (or yellow ore), erubescite (or purple ore), and chalcocite (or grey ore) are the most important. Iron pyrites generally carries copper and is frequently associated with the above-mentioned minerals. These are all attacked by nitric acid. They nearly all contain a small quantity of organic matter, and frequently considerable quantities of lead, zinc, silver, gold, arsenic, bismuth, &c.
The copper ores are often concentrated on the mine before being sent into the market, either by smelting, when the product is a regulus or matte, or by a wet method of extraction, yielding cement copper or precipitate. A regulus is a sulphide of copper and iron, carrying from 30 to 40 per cent, of copper. A precipitate, which is generally in the form of powder, consists mainly of metallic copper. Either regulus or precipitate may be readily dissolved in nitric acid.
Copper forms two classes of salts, cuprous and cupric. The former are pale coloured and of little importance to the assayer. They are easily and completely converted into cupric by oxidising agents. Cupric compounds are generally green or blue, and are soluble in ammonia, forming deep blue solutions.
DRY ASSAY
That, for copper, next after those for gold and silver, holds a more important position than any other dry assay. The sale of copper ores has been regulated almost solely in the past by assays made on the Cornish method. It is not pretended that this method gives the actual content of copper, but it gives the purchaser an idea of the quantity and quality of the metal that can be got by smelting. The process is itself one of smelting on a small scale. As might be expected, however, the assay produce and the smelting produce are not the same, there being a smaller loss of copper in the smelting. The method has worked very well, but when applied to the purchase of low class ores (from which the whole of the copper is extracted by wet methods) it is unsatisfactory. The following table, which embodies the results of several years’ experience with copper assays, shows the loss of copper on ores of varying produce. The figures in the fourth column show how rapidly the proportion of copper lost increases as the percentage of copper in the ore falls below 30 per cent. For material with more than 30 per cent, the proportion lost is in inverse proportion to the copper present.
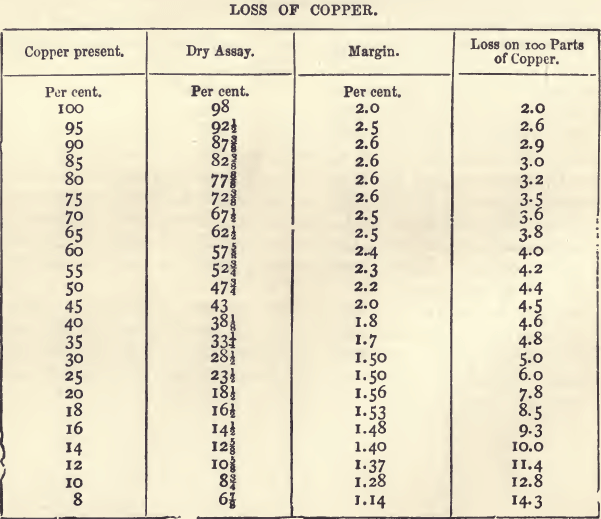
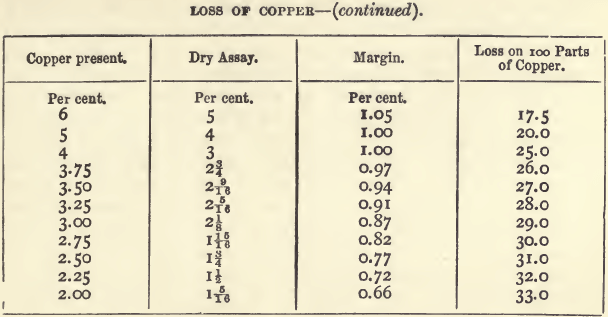
The wet assay being known, the dry assay can be calculated with the help of the above table by deducting the amount in the column headed “ margin ” opposite the corresponding percentage. For example, if the wet assay gives a produce of 17.12 percent., there should be deducted 1.5 ; the dry assay would then be 15.62, or, since the fractions are always expressed in eighths, 15 5/8. With impure ores, containing from 25 to 50 per cent, of copper, the differences may be perhaps ¼ greater.
Wet methods are gradually replacing the dry assay, and it is probable that in the future they will supersede it; for stock-taking, and the various determinations required in smelting works and on mines, they are generally adopted, because they give the actual copper contents, and since it is obvious that a knowledge of this is more valuable to the miner and smelter. Moreover, the working of the dry method has been monopolised by a small ring of assayers, with the double result of exciting outside jealousy and, worse still, of retarding the development and improvement of the process.
The principal stages of the dry assay are : (1) the concentration of the copper in a regulus; (2) the separation of the sulphur by calcining; (3) the reduction of the copper by fusion ; and (4) the refining of the metal obtained.
The whole of these operations are not necessary with all copper material. Ores are worked through all the stages; with mattes, the preliminary fusion for regulus is omitted ; precipitates are simply fused for coarse copper, and refined; and blister or bar coppers are refined, or, if very pure, subjected merely to washing.
The quantity of ore generally taken is 400 grains, and is known as “ a full trial ” ; but for rich material, containing more than 50 per cent, of copper, “ a half trial,” or 200 grains, is used.
Fusion for Regulus
The ore (either with or without a previous imperfect roasting to get rid of any excess of sulphur) is mixed with borax, glass, lime, and fluor spar; and, in some cases, with nitre, or iron pyrites, according to the quality of the ore. The mixture is placed in a large Cornish crucible, and heated as uniformly as possible in the wind furnace, gradually raising the temperature so as to melt down the charge in from 15 to 20 minutes. The crucible is removed and its contents poured into an iron mould. When the slag is solid, it is taken up with tweezers and quenched in water. The regulus is easily detached from the slag. It should be convex above and easily broken, have a reddish brown colour, and contain from 40 to 60 per cent, of copper. A regulus with more than this is “ too fine,” and with less “ too coarse.” A regulus which is too fine is round, compact, hard, and of a dark bluish grey on the freshly broken surface. A coarse regulus is flat and coarse grained, and more nearly resembles sulphide of iron in fracture and colour.
If an assay yields a regulus “ too coarse,” a fresh determination is made with more nitre added, or the roasting is carried further. With low class ores a somewhat coarse regulus is an advantage. If, on the other hand, the regulus is too fine, less nitre or less roasting is the remedy. With grey copper ores and the oxidised ores, iron pyrites is added.
Calcining the Regulus
It is powdered in an iron mortar and transferred to a small Cornish crucible, or (if the roasting is to be done in the muffle) to a roasting dish or scorifier. The calcining is carried out at a dull red heat, which is gradually increased. The charge requires constant stirring at first to prevent clotting, but towards the end it becomes sandy and requires less attention. If the temperature during calcination has been too low sulphates are formed, which are again reduced to sulphides in the subsequent fusion. To prevent this the roasted regulus is recalcined at a higher temperature, after being rubbed up with a little anthracite. The roasted substance must not smell of burning sulphur when hot. It is practically a mixture of the oxides of copper and iron.
Fusion for Coarse Copper
The calcined regulus is mixed with a flux consisting of borax and carbonate of soda, with more or less tartar according to its weight. Some “ assayers ” use both tartar and nitre, the former of course being in excess. The charge is returned to the crucible in which it was calcined, and is melted down at a high temperature, and, as soon as tranquil, poured. When solid it is quenched and the button of metal separated.
The slag is black and glassy. The small quantity of copper which it retains is recovered by a subsequent “ cleaning,” together with the slags from the next operation.
The button of “ coarse copper ” obtained must be free from a coating of regulus. It will vary somewhat in appearance according to the nature and quantity of the impurities.
Refining the Coarse Copper
The same crucible is put back in the furnace, deep down and under the crevice between the two bricks. When it has attained the temperature of the furnace the coarse copper is dropped into it and the furnace closed. The copper will melt almost at once with a dull surface, which after a time clears, showing an “ eye.” Some refining flux is then shot in from the scoop (fig. 48), and, when the assay is again fluid, it is poured. When cold the button of metal is separated.

The button of “ fine ” copper is flat or pitted on its upper surface, and is coated with a thin orange film ; it must have the appearance of good copper. If it is covered with a red or purple film, it is overdone or “ burnt.” If, on the other hand, it has a rough, dull appearance, it is not sufficiently refined. Assays that have been “burnt” are rejected. Those not sufficiently fine are treated as “ coarse copper,” and again put through the refining operation.
Cleaning the Slags
These are roughly powdered and re-fused with tartar, etc., as in the fusion for coarse copper. The button of metal got is separated (if big enough refined) and weighed.
The details of the process are slightly varied by different assayers: the following will be good practice for the student.
Determination of Copper in Copper Pyrites
Powder, dry, and weigh up 20 grams of the ore. Mix with 20 grams each of powdered lime and fluor, 15 grams each of powdered glass and borax, and 5 or 10 grams of nitre. Transfer to a large Cornish crucible and fuse under a loose cover at a high temperature for from 15 to 20 minutes. When fluid and tranquil pour into a mould. When the slag has solidified, but whilst still hot, quench by dipping two or three times in cold water. Avoid leaving it in the water so long that it does not dry after removal. When cold separate the button, or perhaps buttons, of regulus by crumbling the slag between the fingers. See that the slag is free from regulus. It should be light coloured when cold and very fluid when hot. Reject the slag.
Powder the regulus in a mortar and transfer to a small crucible. Calcine, with occasional stirring, until no odour of sulphurous oxide can be detected. Shake back into the mortar, rub up with about 1 gram of powdered anthracite, and re-calcine for 10 minutes longer.
Mix the calcined regulus with 10 grams of tartar, 20 grams of soda, and 3 grams of borax; and replace in the crucible used for calcining. Fuse at a bright red heat for 10 or 15 minutes. Pour, when tranquil.
As soon as solid, quench in water, separate the button of copper, and save the slag.
To refine the copper a very hot fire is wanted, and the fuel should not be too low down in the furnace. Place the crucible well down in the fire and in the middle of the furnace. The same crucible is used, or, if a new one is taken, it must be glazed with a little borax. When the crucible is at a good red heat, above the fusing point of copper, drop the button of copper into it, and close the furnace. Watch through the crevice, and, as soon as the button has melted and appears clear showing an eye, shoot in 10 grams of refining flux, close the furnace, and, in a few minutes, pour; then separate the button of copper. Add the slag to that from the coarse copper fusion, and powder. Mix with 5 grams of tartar, 0.5 gram of powdered charcoal, and 2 grams of soda. Fuse in the same crucible, and, when tranquil, pour; quench, and pick out the prills of metal.
If the copper thus got from the slags is coarse looking and large in amount, it must be refined; but, if small in quantity, it may be taken as four-fifths copper. The combined results multiplied by five give the percentage of copper.
The refining flux is made by mixing 3 parts (by measure) of powdered nitre, 2½ of tartar, and 1 of salt. Put in a large crucible, and stir with a red-hot iron until action has ceased. This operation should be carried out in a well-ventilated spot.
For pure ores in which the copper is present, either as metal or oxide, and free from sulphur, arsenic, &c., the concentration of the copper in a regulus may be omitted, and the metal obtained in a pure state by a single fusion. It is necessary to get a fluid neutral slag with the addition of as small an amount of flux as possible. The fusion should be made at a high temperature, so as not to occupy more than from 20 to 25 minutes. Thirty grams of ore is taken for a charge, mixed with 20 grams of cream of tartar, and 10 grams each of dried borax and soda. If the gangue of the ore is basic, carrying much oxide of iron or lime, silica is added, in quantity not exceeding 10 grams. If, on the other hand, the gangue is mainly quartz, oxide of iron up to 7 grams must be added.
Example.—Twenty grams of copper pyrites, known to contain 27.6 per cent, of copper, gave by the method first described 5.22 grams of copper, equalling 26 1/8- per cent. Another sample of 20 grams of the same ore, calcined, fused with 40 grams of nitre, and washed to ensure the removal of arsenic and sulphur, and treated according to the second method, gave a button weighing 5.27 grams, equalling 26 3/8 per cent. The ore contained a considerable quantity of lead. Lead renders the assay more difficult, since after calcination it remains as lead sulphate, and in the fusion for coarse copper reappears as a regulus on the button.
Estimation of Moisture
The Cornish dry assayer very seldom makes a moisture determination. He dries the samples by placing the papers containing them on the iron plate of the furnace.
It is well known that by buying the copper contents of pyrites by Cornish assay, burning off the sulphur, and converting the copper into precipitate, a large excess is obtained.
NOTES ON THE VALUATION OF COPPER ORES.
Closely bound up with the practice of dry copper assaying is that of valuing a parcel of copper ore. The methods by which the valuation is made have been described by Mr. Westmoreland, and are briefly as follows :—The produce of the parcel is settled by two assayers, one acting for the buyer, the other for the seller; with the help, in case of non-agreement, of a third, or referee, whose decision is final. The dry assayers who do this are in most cases helped, and sometimes, perhaps, controlled, by wet assays made for one or both of the parties in the transaction.
In the case of “ ticketing,” the parcels are purchased by the smelters by tender, and the value of any particular parcel is calculated from the average price paid, as follows:—The “ standard,” or absolute value of each ton of fine copper in the ore, is the price the smelters have paid for it, plus the returning charges or cost of smelting the quantity of ore in which it is contained. The value of any particular parcel of ore is that of the quantity of fine copper it contains, calculated on this standard, minus the returning charges. The ton consists of 21 cwts., and it is assumed that the “ settled ” produce is the actual yield of the ore.
If at a ticketing in Cornwall 985 tons of ore containing 63.3 tons of fine copper (by dry assay) brought £2591 12s., the standard would be £83 15s. This is calculated as follows:— The returning charge is fixed at 55s. per ton of ore. This on 985 tons will amount to £2708 15s. Add this to the actual price paid, and there is got £5300 as the value of the fine copper present. The weight of copper in these 985 tons being 63.3 tons, the standard is £5300÷63.3, or £83 15s. (nearly).
The value of a parcel of 150 tons of a 6 per cent, ore on the same standard would be arrived at as follows:—The 150 tons at 6 per cent, would contain 9 tons (150 x 6 ÷ 100) of fine copper. This, at £83 15s. per ton, would give £733 15s. From this must be deducted the returning charges on 150 tons of ore at 55s. per ton, or £412 10s. This leaves £341 5s. as the value of the parcel.
At Swansea the returning charge is less than in Cornwall, and varies with the quality of the ore. This appears equitable, since in smelting there are some costs which are dependent simply on the number of tons treated, and others which increase with the richness. The returning charge then is made up of two parts, one fixed at so much (12s. 2d.) per ton of ore treated, and the other so much (3s. 9d.) per unit of metal in the ore. In this way the returning charge on a ton of ore of 8¾ produce would be 12s. 2d. + (8¾ x 3s. 9d.), or £2 5s.
If, for example, Chili bars, containing 96 per cent, of copper, bring £50 per ton, the standard is £71 9s. 4d. It is got at in this way. The returning charge on a 96 per cent, ore is 12s. 2d. + (96 x 3s. 9d.), or £18 12s. 2d. This added to £50 gives £68 12s. 2d., and this multiplied by 100 and divided by 96 (100 tons of the bars will contain 96 tons of fine copper) will give £71 9s. 4d.
The price of 100 tons of pyrites, containing 2¼ per cent, of copper by dry assay, would be got on this standard as follows :— The parcel of ore would contain 2¼ tons of copper. This multiplied by the standard gives £160 16s. 0d. From this must be deducted the returning charge, which for 1 ton of ore of this produce would be 12s. 2d.+ (2¼ x 3s. 9d.) or £1 os. 7d., and on the 100 tons is £102 18s. 4d. This would leave £57 17s. 10d. as the price of the parcel, or 11s. 7d. per ton. This would be on the standard returning charge of 45s. (for 8¾ per cent, ore); if a smaller returning charge was agreed on, say 38s., the difference in this case, 7s., would be added to the price per ton.
WET METHODS
The solubility of the ores of copper in acid has already been described, but certain furnace products, such as slags, are best opened up by fusion with fusion mixture and a little nitre.
The method of dissolving varies with the nature of the ore. With 5 grams of pyrites, a single evaporation with 20 c.c. of nitric acid will give a residue completely soluble in 30 c.c. of hydrochloric acid. If the ore carries oxide of iron or similar bodies, these are first dissolved up by boiling with 20 c.c. of hydrochloric acid, and the residue attacked by an addition of 5 c.c. of nitric. When silicates decomposable by acid are present, the solution is evaporated to dryness to render the silica insoluble; the residue extracted with 30 c.c. of hydrochloric acid, and diluted with water to 150 c.c. It is advisable to have the copper in solution as chloride. To separate the copper, heat the solution nearly to boiling (best in a pint flask), and pass a rapid current of sulphuretted hydrogen for four or five minutes until the precipitate settles readily and the liquid smells of the gas. When iron is present it will be reduced to the ferrous state before the copper sulphide begins to separate. The copper appears as a brown coloration or black precipitate according to the quantity present. Filter through a coarse filter, wash with hot water containing sulphuretted hydrogen, if necessary. Wash the precipitate back into the flask, boil with 10 c.c. of nitric acid, add soda till alkaline, and pass sulphuretted hydrogen again. Warm and filter, wash and redissolve in nitric acid, neutralise with ammonia add ammonic carbonate, boil and filter. The copper freed from impurities will be in the solution. Acidulate and reprecipitate with sulphuretted hydrogen. When the nature of the impurities will allow it, this process may be shortened to first filtering off the gangue, then precipitating with sulphuretted hydrogen and washing the precipitate on the filter first with water and then with ammonium sulphide.
Having separated the copper as sulphide, its weight is determined as follows. Dry and transfer to a weighed porcelain crucible, mix with a little pure sulphur, and ignite at a red heat for 5 or 10 minutes in a current of hydrogen. Allow to cool while the hydrogen is still passing. Weigh. The subsulphide of copper thus obtained contains 79.85 per cent, of copper; it is a greyish-black crystalline mass, which loses no weight on ignition if air is excluded.
Copper may be separated from its solutions by means of sodium hyposulphite. The solution is freed from hydrochloric and nitric acids by evaporation with sulphuric acid; diluted to about a quarter of a litre; heated nearly to boiling; and treated with a hot solution of sodium hyposulphite (added a little at a time) until the precipitate settles and leaves the solution free from colour. The solution contains suspended sulphur. The precipitate is easily washed, and under the proper conditions the separation is complete, but the separation with sulphuretted hydrogen is more satisfactory, since the conditions as to acidity, &c., need not be so exact.
Zinc or iron is sometimes used for separating copper from its solutions, but they are not to be recommended.
VOLUMETRIC PROCESSES
There are two of these in use, one based on the decolorising effect of potassic cyanide upon an ammoniacal copper solution, and the other upon the measurement of the quantity of iodine liberated from potassic iodide by the copper salt. The cyanide process is the more generally used, and when carefully worked, “ on certain understood and orthodox conditions,” yields good results; but probably there is no method of assaying where a slight deviation from these conditions so surely leads to error. An operator has no difficulty in getting concordant results with duplicate assays; yet different assayers, working, without bias, on the same material, get results uniformly higher or lower; a difference evidently due to variations in the mode of working. Where a large number of results are wanted quickly it is a very convenient method. The iodide process is very satisfactory when worked under the proper conditions.
Copper CYANIDE Assay METHOD
The process is based upon the facts—(1) that when ammonia is added in excess to a solution containing cupric salts, ammoniacal copper compounds are formed which give to the solution a deep blue colour; and (2) that when potassic cyanide is added in sufficient quantity to such a solution the colour is removed, double cyanides of copper and potassium or ammonium being formed. In the explanation generally given the formation of cuprous cyanide is supposed ; but in practice it is found that one part of copper requires rather more than four parts of cyanide, which agrees with the former, rather than the latter, explanation.
Reliance on the accuracy of the process cannot rest upon the supposition that the cyanide required for decoloration is proportional to the copper present, for varying quantities of ammonia salts, ammonia and water, and differences of temperature have an important effect. The results are concordant and exact only when the cyanide is standardised under the same conditions as it is used. It is best to have the assay solution and that used for standardising as nearly as possible alike, and to titrate the two solutions side by side. This demands an approximate knowledge of the quantity of copper contained in the ore and a separation of the bulk of the impurities.
For the titration there is required a standard solution of potassium cyanide made by dissolving 42 grams of the salt, known to dealers as Potassium Cyanide (Gold), in water and diluting to one litre: 100 c.c. of this will be about equivalent to one gram of copper. For poor ores the solution may conveniently be made half this strength.
The solution of the ore and the separation of the copper as sulphide are effected in the same ways as have been already described for electrolysis. Similarly, too, the sulphide is attacked with 15 c.c. of nitric acid and the assay boiled down to 10 c.c. Add 20 c.c. of water and warm, filter into a pint flask, wash well with water, and dilute to about 150 c.c.; add 30 c.c. of dilute ammonia, and cool.
Prepare a standard by dissolving a quantity of electrotype copper (judged to be about the same as that contained in ths assay) in 20 c.c. of water and 10 c.c. of nitric acid, boil off the nitrous fumes, and dilute to 150 c.c.: add 30 c.c. of dilute ammonia and cool.
Fill a burette with the standard cyanide solution. The burette with syphon arrangement, figured on page 52, is used. A number of titrations can be carried on at the same time provided the quantity of copper present in each is about the same. This is regulated in weighing up the ore. The flasks must of course be marked, and should be arranged in series on a bench in front of a good light and at such a height that the liquid can be looked through without stooping. Supposing about 50 c.c. of cyanide will be required, 30 c.c. should be run into each, and each addition be recorded as soon as made; then run 15 c.c. into each.
The solutions will now probably show marked differences of tint; add 1 c.c; of cyanide to the lighter ones and more to the darker, so as to bring the colours to about the same depth of tint. They should all be of nearly equal tint just before finishing. At the end add half a c.c, at a time until the colours are completely discharged. A piece of damp filter paper held between the light and the flask assists in judging the colour when nearly finished.
Overdone assays show, a straw yellow colour which deepens on standing.
The following will illustrate the notes recorded of five such assays and one standard :—

Three grams of ore were taken, and the standard contained 0.480 gram of copper. In this series the difference of half a c.c. means about 0.15 per cent, on the ore; with a little practice it is easy to estimate whether the whole or half of the last addition should be counted.
To get satisfactory results, the manner of finishing once adopted must be adhered to.
The following experiments show the effect of variation in the conditions of the assay :—Use a solution of copper nitrate, made by dissolving 10 grams of copper in 50 c.c. of water and 35 c.c. of nitric acid, and diluting to a litre. 100 c.c. = 1 gram of copper.
Effect of Varying Temperature
In these experiments 20 c.c. of copper nitrate were used, with 10 c.c. of nitric acid, 30 c.c. of dilute ammonia, and water to 200 c.c. The results were—

The temperature is that of the solution before titrating. These show the importance of always cooling before titrating, and of titrating the assay and standard at the same temperature.
Effect of Varying Bulk
The quantities of copper, acid, and ammonia were the same as in the last-mentioned experiments. The results were:—
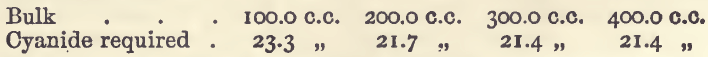
These show that large variations in bulk must be avoided.
Effect of Varying Ammonia
The quantities of copper and acid were the same as in the series of experiments last noticed. The bulk was 200 c.c. The results were:—
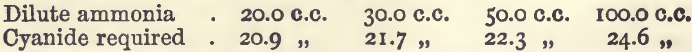
Effect of Varying Acid
The quantities of copper and water were the same as in the last-noticed set of experiments: 30 c.c. of dilute ammonia were used.

On adding nitric acid to the solution it combines with a portion of the ammonia to form ammonic nitrate; it will be seen from the last series of experiments that the lessening of the amount of free ammonia will decrease the quantity of cyanide required; but, on the other hand, the ammonic nitrate which is at the same time formed will increase the amount required; under the conditions of the assay these two effects neutralise each other, and such differences in the quantity of acid as are likely to occur are unimportant.
Effect of Varying Ammonic Salts
The quantities of copper, water, and ammonia were the same as in the last mentioned set of experiments, but no nitric acid was used.
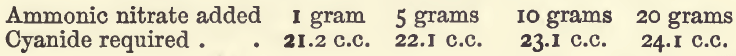
These show that combined ammonia seriously affects the titration, and that the principle sometimes recommended of neutralising the acid with ammonia, and then adding a constant quantity of ammonia, is not a good one, because there is then an interference both by the ammonia and by the variable quantity of ammonic salts.
The same quantity of combined ammonia has the same effect, whether it is present as sulphate, nitrate, chloride, or acetate, as the following experiments show. Four lots of 20 c.c. of “ copper nitrate ” were taken, and 20 c.c. of dilute ammonia added to each. These were carefully neutralised with the respective acids, rendered alkaline with 30 c.c. more of ammonia, cooled, diluted to bulk, and titrated. The results were :—

Effect of Foreign Salts
Sulphates, nitrates and chlorides of sodium or potassium have no action, whilst the hydrates, carbonates, bicarbonates, sulphites, and nitrites have an important effect. The interference of ammonic salts has already been shown.
Salts of silver, zinc, and nickel react with cyanide just as copper does, and consequently interfere. Ferrous salts are sure to be absent, and ferric salts yield ferric hydrate with the ammonia, which is not acted on by the cyanide, but, owing to its bulkiness, it settles slowly; this lengthens the time required for titration, and so modifies the manner of working. An assay should not be worked with ferric hydrate present) unless the standard contains about the same amount of it. On mines it is often inconvenient to separate the copper by means of sulphuretted hydrogen: hence it is customary to titrate without previous separation. In this case, instead of standardising the cyanide with electrotype copper, a standard ore should be used. This should be an ore (of the same kind as those being assayed) in which the copper has been carefully determined.
Effect of Varying Copper
In these experiments 10 c.c. of nitric acid, 30 c.c. of ammonia, and water to 200.C.C. were used.
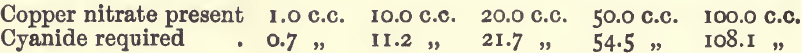
These results show that under the conditions laid down the various causes of disturbance nearly neutralise one another, and the results within a fair range are practically proportional.
Determination of Copper in Copper Pyrites
Weigh up 2 grams of the dried and powdered ore, and place in an evaporating dish about four inches in diameter. Cover with 20 c.c. of nitric acid and put on a hot plate. Evaporate to dryness without further handling. Allow to cool and take up with 30 c.c. of hydrochloric acid, boil, dilute, and transfer to a pint flask, filtering if necessary. Make up the bulk with the washings to about 150 c.c. Precipitate with sulphuretted hydrogen, filter, and wash back the precipitate into the flask. Add 15 c.c. of nitric acid, and boil down rapidly to 10 c.c. Dilute, add 30 c.c. of dilute ammonia, make up to 150 c.c., and cool. For the standard, weigh up 0.5 gram of copper, more or less, according to the quantity judged to be present in the assay. Dissolve in 20 c.c. of dilute nitric acid, boil off nitrous fumes, add 30 c.c. of dilute ammonia, make up to the same bulk as that of the assay, and cool. Titrate the two solutions side by side and as nearly as possible in the same manner.
Since the assay solution is often turbid from the presence of small quantities of lead and of iron from incomplete washing, and since this slight precipitate is very slow in settling, the standard can hardly be compared strictly with the assay. This can be counteracted by precipitating in both solutions a mixture of ferric and aluminic hydrates, which settles readily and leaves the supernatant liquor clear. To effect this, boil the nitric acid solutions with 30 c.c. of a solution containing 15 grams each of alum and ferrous sulphate to the litre. In an actual determination 2 grams of the ore were taken and compared with 0.5 gram of copper. The assay required 57.7 c.c. of cyanide and the standard 52.5 c.c.
52.5 : 0.5 :: 57.7 : 0.5495
This on 2 grams of ore =27.47 per cent; the same sample by electrolysis gave 27.60 per cent, of copper.
Determination without Previous Separation
Dissolve up 2 grams as before, but, instead of passing sulphuretted hydrogen, add 30 c.c. of dilute ammonia, shake well, and cool. Prepare a standard by dissolving 0.5 gram of copper in 1 c.c. of nitric acid, add 0.6 gram of iron in the form of ferric chloride and 20 c.c. of hydrochloric acid, dilute to about 150 c.c., add 30 c.c. of dilute ammonia, and cool. Titrate the two solutions side by side. In a determination on the sample last used, 58 c.c. were required for the assay and 53 c.c. for the standard, which indicates 27.3 per cent, of copper.
This method of working is somewhat rough.
IODIDE METHOD
This is based upon the fact that when potassic iodide in excess is added to a strong solution of a cupric salt in a faintly acid solution, cuprous iodide is formed and an equivalent of iodine liberated. The iodine is measured by titrating with a solution of sodium hyposulphite, using starch paste as indicator. The iodine is soluble in the excess of potassium iodide, forming a deep brown solution; the hyposulphite is added until this brown colour is almost removed. Starch paste is then added, and strikes with the remaining iodine a dirty blue colour. The addition of the “ hypo” is continued until the blue colour is discharged. The end reaction is sharp ; a drop is sufficient to complete it.
As regards the titration, the process leaves little to be desired ; the quantity of “hypo” required is strictly proportional to the copper present, and ordinary variations in the conditions of working are without effect. The presence of salts of bismuth masks the end reaction because of the strong colour imparted to the solution by the iodide of bismuth. Under certain conditions there is a return of the blue colour in the assay solution after the finishing point has apparently been reached, which is a heavy tax on the patience and confidence of the operator. This is specially apt to occur when sodium acetate is present, although it may also be due to excessive dilution.
The standard “ hypo” solution is made by dissolving 39.18 grams of the crystallised salt (Na2S2O2.5H2O) in water and diluting to one litre. One hundred c.c. will equal one gram of copper.
The starch solution is made by mixing 1 gram of starch into a thin paste with cold water, pouring it into 200 c.c. of boiling water, and continuing the boiling for a minute or so. The solution must be cold before use, and about 2 c.c. is used for each assay. It should not be added until the bulk of the iodine has been reduced.
To standardise the “ hypo,” weigh up 0.3 or 0.4 gram of pure copper, dissolve in 5 c.c. of dilute nitric acid, boil off nitrous fumes, and dilute with an equal bulk of cold water. Add “ soda ” solution until a permanent precipitate is obtained, and then 1 c.c. of acetic acid. This should yield a clear solution. Fill an ordinary burette with the “ hypo.” Add 3 grams of potassium iodide crystals to the copper solution, and, when these are dissolved, dilute to 100 c.c. with water. Run in the “ hypo ” solution rather quickly until the brown colour is nearly discharged—i.e., to within 3 or 4 c.c. of the finish. Add 2 c.c. of the starch solution, and continue the addition of the “ hypo ” a few drops at a time until the tint suddenly changes to a cream colour. The blue colour must not return on standing three or four minutes. Calculate the standard in the usual way.
In assaying ores, the copper is dissolved and separated with sulphuretted hydrogen as in the other processes, but the sulphide should be washed more completely to ensure the absence of iron salts.
The following experiments show the effect of variation in the conditions of the assay. Use a solution of copper sulphate containing 39.38 grams of copper sulphate crystals (CuSO4.5H2O) in the litre. 100 c.c. equal 1.00 gram of copper.
Effect of Varying Temperature
The assay after the addition of the potassic iodide must be kept cold, else iodine may be volatilised.
Effect of Varying Potassium Iodide
In various descriptions of the process the amount of iodide required is variously stated at from “a few crystals” to as much as 10 grams. The proportion required by theory for 1 gram of copper is a little over 5 grams: an excess, however, is required to keep the liberated iodine in solution. On economic grounds this excess should not be extravagant; if the student uses 10 parts of the iodide for each part of copper in the assay he will have sufficient. In the experiments there were used 20 c.c. of the copper sulphate, with varying amounts of potassic iodide, and the following results were got:—
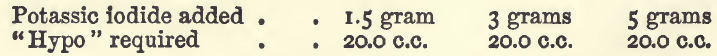
In these the iodide was added direct to the solution containing the copper, which was afterwards diluted to 100 c.c. and titrated.
In another series the iodide was added after the dilution to 100 c.c., and the results were :—
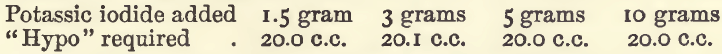
Effect of Varying Bulk.—In these experiments, 20 c.c. of copper sulphate were taken, 3 grams of potassic iodide added, and also water to the required bulk.
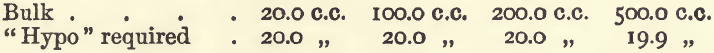
In the last of these experiments the colour was discharged at 18 c.c., but gradually returned until 19.9 c.c. had been run in. It will be seen that considerable variation in bulk does not interfere.
Effect of Acetic Acid
These experiments were like the last series mentioned, but the bulk was 100 c.c., and varying amounts of acetic acid were added.
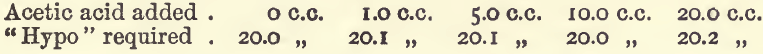
Acetic acid, then, does not interfere to any serious extent.
Effect of Varying Sodium Acetate
These experiments were like those last mentioned, but without acetic acid, and with varying amounts of sodium acetate.
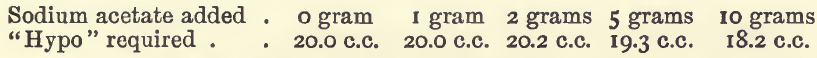
In the 5 grams experiment, when the finishing point had been apparently reached the colour slowly returned ; but as the results generally on titrating were not satisfactory a repetition of the experiment was made with the addition of 5 c.c. of acetic acid, which gave an equally bad result.
Effect of Foreign Salts
The conditions of these experiments were the same as before. The salts were added and dissolved before the addition of the potassium iodide. Using 5 grams (or in the case of the acids, 5 c.c.), the results were as follows:—

The low result with the sulphate of soda was evidently due to the formation of a sparingly soluble double salt, which removed copper from the solution; on adding a little acetic acid the full amount of “ hypo” was required. The effect of the presence of certain metals is important. The method of determining it was to add the substance to the solution containing the copper, and partly precipitate with soda solution; then treating with 1 c.c. of acetic acid, adding the iodide, and proceeding as before.
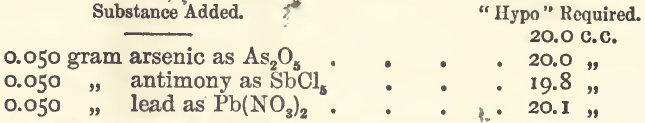
A similar experiment with 0.050 gram of bismuth nitrate could not be determined, the end-reaction being masked. Bismuth iodide is soluble in potassic iodide, forming a brown solution, the colour of which is very similar to that produced by iodine; and although it does not strike a blue colour with starch, “ hypo” has an action on it.
A similar experiment with 0.050 gram of iron as ferric chloride required 22.3 c.c. of “ hypo,” and the colour returned on standing. This shows that ferric acetate liberates iodine under the conditions of the assay. Trying to counteract this, by adding to a similar solution 0.5 gram of phosphate of soda dissolved in a little water, 19.7 c.c. of “hypo” were required instead of 20.0, but the assay showed signs of returning colour.
In standardising, the same result was obtained, whether the copper was present as nitrate or sulphate before neutralising.
Effect of Varying Copper.—With the same conditions as before, but with varying amounts of copper and a proportionally increasing quantity of iodide, the results were :—
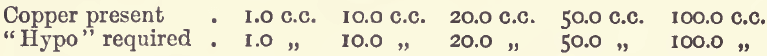
showing the results to be exactly proportional.
Determination of Copper in Copper Pyrites
Take 2 grams of the dried and powdered ore and treat in a porcelain dish with 20 c.c. of nitric acid, and evaporate to dryness. Take up with 30 c.c. of hydrochloric acid, dilute, and transfer to a pint flask; make up with water to 200 c.c., warm, and pass sulphuretted hydrogen to excess. Filter, and wash the precipitate with water acidified with sulphuric acid. Wash the precipitate back into the flask, and dissolve with 15 c.c. of nitric acid. Evaporate almost to dryness ; add 20 c.c. of water, and boil till free from nitrous fumes; filter off the sulphur and gangue; neutralise with soda, avoiding excess ; add 1 or 2 c.c. of acetic acid, and shake till clear. Add 5 grams of potassium iodide, dilute to 100 c.c., and titrate. The following is an example:—

which is equal to 27.5 per cent, of copper.
COLORIMETRIC PROCESS
This is based on the blue coloration of ammoniacal copper solutions. The quantity of copper in 100 c.c. of the assay solution should not be more than 15 milligrams, or less than half a milligram. It is not so delicate as most other colorimetric methods, but nevertheless is a very useful one.
The manner of working is the same as that described under iron.
Standard Copper Solution.—Weigh up 0.5 gram of electrotype copper, dissolve in 10 c.c. of nitric acid, boil off nitrous fumes, and dilute to 1 litre. 1 c.c. = 0.5 milligram.
In nearly all cases it will be necessary to separate the copper with sulphuretted hydrogen from a solution of about 5 grams of the material to be assayed. The filter paper containing the sulphide (and, probably, much sulphur) is dried and burnt. The ashes are dissolved in 5 c.c. of dilute nitric acid, 10 c.c. of dilute ammonia added, and the solution filtered through a coarse filter into a Nessler tube, washing the paper with a little dilute ammonia.
The estimation of the colour and calculation of the result are made in the way described on page 44.
The effect of varying conditions on the assay may be seen from the following experiments.
Effect of Varying Temperature.—The effect of increased temperature is to slightly decrease the colour, but this can only be observed when a fair quantity of copper is present.

Effect of Varying Ammonia.—The solution must, of course, contain free ammonia; about 5 c.c. of dilute ammonia in 50 c.c. bulk is the quantity to be used in the experiments. A larger quantity affects the results, giving lower readings and altering the tint. With small quantities of ammonia the colour approaches a violet; with larger, a sky-blue.
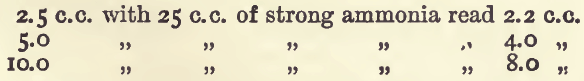
Effect of Ammonic Salts.—The following table shows the results after addition of ammonic salts :—

These show that sulphates should be avoided, and either nitrate or chloride solutions be used in the standard as well as in the assay.
Determination of Copper in a Manganese Ore
Treat 3 grams of the ore with 20 c.c. of hydrochloric acid, and evaporate to dryness. Take up with 10 c.c. of hydrochloric acid; dilute to about 200 c.c., and pass sulphuretted hydrogen until the solution smells of the gas; filter, burn, take up with 5 c.c. of dilute nitric acid, add 10 c.c. of dilute ammonia, and filter into the Nessler tube, and make up with the washings to 50 c.c. Into the “standard” tube put 5 c.c. of dilute nitric acid and 10 c.c. of dilute ammonia. Make up to nearly 50 c.c. with water, and run in the standard copper until the colours are equal. In a determination 4 c.c. ( = 2. o milligrams of copper) were required; this in 3 grams of ore = 0.07 per cent.
Determination of Copper in “ Black Tin”
Weigh up 3 grams of the dried ore, boil with 10 c.c. of hydrochloric acid, and afterwards add 1 c.c. of nitric; boil off nitrous fumes, evaporate to about 5 c.c., dilute to 50 c.c., add 20 c.c. of dilute ammonia; stir, and filter. If much iron is present, dissolve the precipitate of ferric hydrate in acid, and reprecipitate with ammonia. Mix the two filtrates, and dilute to 100 c.c. Take 50 c.c. for the test. A sample of 3 grams of an ore treated in this way required 5.2 c.c. of standard copper to produce equality of tint. This gives 0.35 per cent.
Determination of Copper in Tin
Weigh up 1 gram of the sample, transfer to an evaporating dish, and cover with 30 c.c. of aqua regia. Warm until the metal has dissolved, then evaporate almost to dryness. Take up with a few c.c. of hydrochloric acid and again evaporate.
Dissolve the residue in 10 c.c. of dilute hydrochloric acid and transfer to a 100 c.c. flask. Add 10 c.c. of dilute ammonia and make up with water to the containing mark.
Filter off 50 c.c. of the solution into a Nessler glass and determine the copper in it colorimetrically.
EXAMINATION OF COMMERCIAL COPPER
Very pure copper can be obtained in commerce, owing to the demand for metal of “ high conductivity ” for electrical purposes, which practically means for metal free from impurities.
Much of the metal sold contains as much as one per cent, of foreign substances, of which arsenic is the most important. The other elements to be looked for are bismuth, lead, antimony, silver, gold, iron, nickel, cobalt, sulphur, and oxygen. In “ blister copper ” (which is the unrefined metal), aluminium, silicon, and phosphorus may be met with.
Oxygen.—All commercial copper carries oxygen; most of it is present as cuprous oxide, which is dissolved by molten copper. The estimation of oxygen is often made “ by difference.” The copper and the other impurities being determined, the rest is assumed to be oxygen. Probably this is nearly correct, but the whole of the oxygen should not be ascribed to cuprous oxide; for any arsenic the metal contained would be present as cuprous arsenite, since arsenide of copper and cuprous oxide could not exist together at the temperature of fusion without interacting. In the report of the analysis, it is best to state the proportion of oxygen thus:—
Oxygen per cent, by difference.
There is a method of determination by fusing 5 or 10 grams in a brasqued crucible, and counting the loss as oxygen; and another method for the determination of cuprous oxide based on the reaction of this substance with nitrate of silver. About 2 grams of silver nitrate, dissolved in 100 c.c. of water, is allowed to act upon 1 gram of the copper in the cold. The precipitate is filtered off, washed thoroughly with water, and the basic salt dissolved and determined colorimetrically.
One part of copper found represents 1.68 part of cuprous oxide, or 0.19 part of oxygen. Copper generally carries from 0.1 to 0.2 per cent, of oxygen.
Silver is found in most samples, but occurs in variable proportions ; when it amounts to 30 ounces per ton it has a commercial value. To determine its amount, dissolve 10 grams of the copper in 35 c.c. of nitric acid and 50 c.c. of water, boil off nitrous fumes, and dilute to about 100 c.c. One or two c.c. of dilute hydrochloric acid (one to 100 of water) are added, stirred in, and the precipitate allowed to settle for twenty-four hours. Filter through a double Swedish paper, dry, burn, and cupel the ashes with one gram of sheet lead.
Ten grams of a sample of copper gave in this way 4.7 milligrams of silver. Ten grams of the same copper, to which 24 milligrams of silver had been added gave 28.2 milligrams.
Gold.—To determine it, dissolve 10, 20, or 50 grams of the sample in 35, 70, or 175 c.c. of nitric acid and an equal volume of water, boil till free from nitrous fumes, and dilute to double its volume. Allow to stand for some time, decant on to a filter, dry, burn, and cupel the ashes with 1 gram of sheet lead. If silver is present, owing to traces of chlorides in the re-agents used, “parting” will be necessary. (See Gold.)
Working in this way on 20 grams of copper, to which 1.8 milligram of gold had been added, a button weighing 2.0 milligrams was obtained.
Antimony, is not a frequent impurity of copper: it can be detected in quantities over o. 1 per cent, by a white residue of Sb2O4 insoluble in nitric acid. With material containing only small quantities of antimony the white oxide does not show itself for some time, but on long-continued boiling it separates as a fine powder. It is best (when looking for it) to evaporate the nitric acid solution to the crystallising point, to add a little fresh nitric acid and water, and then to filter off the precipitate. After weighing it should be examined for arsenic and bismuth.
Lead.—Refined coppers are often free from lead, anything more than traces being seldom found; in coarse coppers it is sometimes present in considerable quantities.
Its presence may be detected in the estimation of the copper electrolytically, the platinum spiral becoming coated with a brown or black deposit of lead dioxide. The depth of colour varies with the lead present, and obviously could be made the basis of an approximate estimation. The colour shows itself within an hour or so, but is best observed when all the copper has been deposited.
Electrolysing a solution of one gram of pure copper, to which 0.5 milligram of lead had been added, the deposit was dark brown; in a similar solution with 1 milligram of lead it was much darker, and with 2 milligrams it was black. Under the conditions of the assay the dioxide cannot be weighed, as it partly dissolves on breaking the current. When lead has been found, its quantity may be estimated by evaporating to dryness the nitric acid solution to which an excess of sulphuric acid has been added, taking up with water, and filtering off and weighing the lead sulphate.
The separation of traces of lead as chromate is a fairly good one. Dissolve 5 grams of the copper in 17 c.c. of nitric acid and an equal volume of water ; boil off nitrous fumes, neutralise with soda, and afterwards acidulate with acetic acid; and dilute to a litre. Add 20 grams of sodium acetate, warm, and precipitate the lead with a dilute solution of potassium chromate. Copper chromate (yellow) may be at the same time thrown down, but it is readily soluble on diluting. Filter off the precipitate; wash it into a beaker and pass sulphuretted hydrogen; oxidise the sulphide and weigh as lead sulphate. Treated in this way 5 grams of copper yielded sulphate of lead equal to 2.0 milligrams of lead. Five grams of the same sample to which 10 milligrams of lead were added gave 11.4 milligrams.
Nickel and Cobalt.—Nickel is always present in larger or smaller quantities in commercial copper, and, perhaps, has an influence on the properties of the metal. It is determined as follows:—Dissolve 10 grams of the copper in 35 c.c. of nitric acid and an equal bulk of water, boil off nitrous fumes and neutralise with soda, add 2 grams of carbonate of soda dissolved in water, boil, and filter. Acidify the filtrate with 2 or 3 c.c. of dilute nitric acid and dilute to 1 or 1½ litres. Pass sulphuretted hydrogen through the cold solution till the copper is all down and the liquid smells of the gas. Filter and evaporate the filtrate to a small bulk, and determine the nickel by electrolysing the solution rendered ammoniacal, or by precipitating as sulphide and weighing as sulphate. (See under Nickel.) The precipitate, after weighing, should be tested for cobalt. If present it is separated with potassium nitrite as described under Cobalt. Ten grams of copper gave 6.0 milligrams of nickel; and another lot of 10 grams of the same copper, to which 10.0 milligrams of nickel had been added, gave 17.2 milligrams.
Sulphur.—The amount of sulphur in refined copper is very small, seldom exceeding 0.005 per cent. In coarse copper, as might be expected, it is found in larger quantities.
In determining it, it is first converted into sulphuric acid, and then precipitated and weighed as barium sulphate. The precipitation cannot be effected from a nitric acid solution. Ten grams of copper are dissolved in nitric acid, as for the other determinations, and then boiled with excess of hydrochloric acid till the nitric acid is completely removed. There is then added a few drops of a dilute solution of baric chloride, and the solution is allowed to stand for some hours. The baric sulphate is filtered off and weighed.
The necessity for precipitating from a hydrochloric acid solution is seen from the following determinations. In each experiment 10 grams of copper was used, and a known weight of sulphur, in the form of copper, sulphate, added.

Bismuth.—Nearly all samples of copper contain bismuth, but only in small quantities. It is best determined colorimetrically as described under Bismuth. The method of concentrating and preparing the solution for colorimetric assay is as follows. Dissolve 10 grams of copper in nitric acid, as in the other determinations; neutralise with soda; add 1 or 1.5 grams of bicarbonate of soda and boil for ten minutes ; filter, dissolve the precipitate in hot dilute sulphuric acid; add sulphurous acid and potassium iodide in excess, and boil till free from iodine. Filter and dilute to 500 c.c. Take 50 c.c. of the yellow solution for the determination. A few c.c. of a dilute solution of sulphurous acid (1 in 100) will prevent the liberation of iodine. The following experiments test the method of separation. Ten grams of copper were treated as above and precipitated with 1.5 gram of “ soda; ” the precipitate contained 0.6 milligram of bismuth ( = 0.006 per cent.). The filtrate treated with another 1.5 gram of “ soda ” gave a precipitate which was free from bismuth. To the filtrate from this was added 1.0 milligram of bismuth, and another fraction was precipitated with 1.5 gram of “ soda.” In this precipitate was found 1.0 milligram of bismuth. To the filtrate another milligram of bismuth was added and the separation with “soda” repeated. The bismuth was separated from this precipitate with ammonic carbonate before determination, and 0.9 milligram was found.
Arsenic.—The proportion of arsenic in copper varies from 0. 01 to 0.75 per cent, whilst in coarse copper it may amount to 2 or even 3 per cent. To determine it, dissolve 5, 10, or 20 grams of the copper (according to the amount of arsenic present) in 18 c.c., 35 c.c., or 70 c.c. of nitric acid, and an equal volume of water. Boil off the nitrous fumes, dilute to 100 c.c. and neutralise with soda; add 1.5 or 2 grams of carbonate of soda dissolved in a little water, and boil. Filter (washing is unnecessary) and dissolve back into the flask with a little dilute hydrochloric acid ; add 30 c.c. of dilute ammonia and 25 c.c. of “magnesia mixture,” and allow to stand overnight. The whole of the arsenic is precipitated as ammonic-magnesic arsenate in one hour, but it is advisable to leave it longer. The precipitate may be dried and weighed, or, better, titrated with uranium acetate. (See Arsenic.) To test this method of separation 10 grams of pure copper were taken and 0.200 gram of arsenic dissolved with it. The arsenic was determined by titration with uranium acetate, and 0.200 gram was found. Two other similar experiments with 0.080 and 0.010 gram of arsenic added, gave 0.079 and 0.012 gram respectively.
Antimony or bismuth may be present without interfering with the titration. With 0.100 gram of antimony and 0.100 gram of arsenic, 0.100 gram of arsenic was found ; and in another case, with 0.100 gram of bismuth and 0.060 gram of arsenic, 0.060 gram was found. In these experiments the antimony and bismuth were present in the assay solution when titrated. For a gravimetric determination they would require to be removed before precipitating with “ magnesia mixture.”
Phosphorus, if present, counts as arsenic in the proportion of 1 to 2.4; but, except in the case of coarse coppers, it is always absent.
Iron, if present, interferes by forming a white flocculent precipitate of ferric arsenate after the addition of the sodium acetate and preliminary to the titration. Each milligram of iron abstracts, in this way, 1.3 milligrams of arsenic.
Iron.—Refined coppers carry traces of iron, varying from 0.001 to 0.01 per cent. It is best determined during the arsenic estimation. The precipitate of the ammonic-magnesic arsenate will contain the whole of the iron as ferric hydrate. On dissolving in hydrochloric acid, neutralising with ammonia, adding 5 c.c. of sodic acetate, diluting, and boiling, it reappears as a white precipitate of ferric arsenate. It is filtered off (the arsenic being estimated in the filtrate), dissolved in warm hydrochloric acid, and determined colorimetically as described under Iron. A series of experiments testing the separation is there given.
Phosphorus.—Refined coppers do not carry phosphorus, although it may be present in “ coarse copper ” up to 1 per cent, or more. In such samples the following method is adopted for the estimation of both phosphorus and arsenic. Dissolve 10 grams of copper and 0.1, 0.2, or 0.3 gram of iron wire (according to the amount of arsenic and phosphorus present) in 35 c.c. of nitric acid and an equal volume of water. Add soda till the free acid is nearly neutralised. Next add a strong solution of sodium acetate, until the solution ceases to darken on further addition, then dilute with water to half a litre. The solution is best contained in a large beaker; it is next heated to the boiling point, and at once removed and allowed to settle. If the precipitate is light coloured it is evidence that sufficient iron has not been added, or, if it is green; from basis copper salts, it shows that the solution was not sufficiently acid. In either case start afresh. Filter off the precipitate and wash with hot water containing a little sodium acetate, dissolve it off the filter with hot dilute hydrochloric acid, add ammonia in excess, and pass sulphuretted hydrogen for five minutes. Warm at about 70° C. for a quarter of an hour. Filter. The clear yellow filtrate contains the arsenic and phosphorus. Add dilute sulphuric acid in excess; filter off the yellow precipitate of sulphide of arsenic, dissolve it in nitric acid, and titrate with uranium acetate, as described under Arsenic.
The filtrate from the sulphide of arsenic is rendered alkaline with ammonia and “ magnesia mixture ” added. The solution is stirred, and allowed to stand overnight. The precipitate of ammonic-magnesic phosphate is filtered off, dissolved, and titrated with uranium acetate, using the same standard solution as is used in the arsenic assay: 0.5 gram of arsenic equals 0.207 gram of phosphorus.
Copper.—The method of determining this has been described under Electrolytic Assay.
In the method of concentration by fractional precipitation with sodic carbonate (which is adopted in most of these determinations) the precipitate will contain all the bismuth, iron, and alumina; the arsenic and phosphorus as cupric arsenate and phosphate; and the greater part of the lead, antimony, and silver. The nickel and cobalt, and the sulphur as sulphuric acid, will remain in solution with the greater part of the copper.
PRACTICAL EXERCISES.
1. According to a wet assay 2 grams of a certain ore contained o. 3650 gram of copper. What would you expect the dry assay produce to be?
2. A standard solution is made by dissolving 25 grams of potassic cyanide and diluting to a litre. Assuming the salt to be 98 per cent, real cyanide, what would 100 c.c. of the solution be equivalent to in grams of copper ?
3. How would you make a solution of “ hypo ” of such strength that 100 c.c. shall equal 0.633 gram of copper ?
4. What weight of ore, containing 17.0 per cent, of copper, would you take in order to get about 0.5 gram of copper in solution for electrolysis ?
5. The solution of copper in nitric acid is effected by the following reaction:—
3Cu + 8HNO3 = 3Cu(NO3)2 + 4H2O + 2NO.
What volume of nitric acid will be required to dissolve 1 gram of copper ?
