The use of revolving barrels for chlorinating ores was perhaps suggested by the old Freiberg method of barrel amalgamation. It has already been mentioned, p. 239, that Dr. Duflos used a revolving barrel in some of his experiments at Breslau, in 1848, and obtained results almost identical with those given by the vat percolation method. He, therefore, preferred the latter as being cheaper. The next mention of revolving barrels seems to have been contained in a patent taken out by Mr. De Lacy in Victoria, in 1864. The process thus patented appears to have been tried there, probably on a small scale, but certainly never passed into general use and was soon forgotten. In 1877, Dr. Howell Mears, of Philadelphia, patented a process which had some points of resemblance to that described by De Lacy. This process was gradually improved in practice, after having been adopted by several mines in the United States, and in particular the improvements introduced by Adolph Thies, in 1881, caused the name of the Thies process to be applied to the amended method of procedure. In 1887 the barrel process was re-introduced with some modifications into Australia by Prof. J. Cosmo Newbery and Mr. C. J. T. Vautin, who applied it to the ore of the Mount Morgan Mine, where it was successfully worked for some years before it was replaced by the vat solution process. In 1889 and succeeding years the barrel process was developed in the United States, and appeared likely to become of great importance. Of late years, however, it has been generally regarded with less favour than the cyanide process.
The Mears Process: In this process, the roasted ore was charged into lead-lined iron cylindrical barrels, together with enough water to make an easily flowing pulp ; chlorine was then forced in under pressure through the hollow trunnion of the barrel, which was revolved until the gold had been dissolved. The pressure of chlorine was stated to be as much as 40 or 50 lbs. to the square inch. The manhole of the barrel was then opened and the ore discharged by gravity into a leaching vat below, where the soluble gold was washed out and precipitated by any of the known methods.
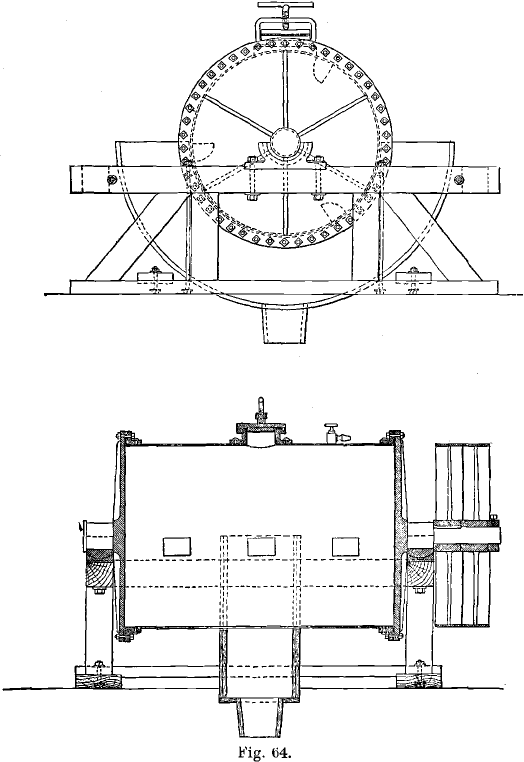
The Thies Process: In this process the chlorine gas was generated inside the barrel itself by means of bleaching powder and sulphuric acid. The method was simplified in this way, and a number of leaky joints dispensed with. Thies found that a moderate pressure of chlorine, of a few pounds to the square inch, was enough. His barrel is shown in Fig. 64. It was about 5 feet long, and held from 2,000 to 2,500 lbs. of ore.
Working Principles of Barrel Chlorination
The barrel is revolved at the rate of six to ten revolutions per minute by a belt and pulley (the latter being fixed on a continuation of the trunnion), or by means of cog-wheels, or by a friction clutch. The ore is kept constantly stirred and tumbled about by the revolution of the barrel, the advantages gained from this course being as follows:
- Every particle of ore is exposed equally to the action of the chlorine.
- There can be no local exhaustion of the solvent near coarse particles of gold.
- It was formerly argued that the gold would be cleaned by attrition, and in particular that silver chloride, formed by the action of chlorine, would be rubbed off the surface of particles of native gold-silver alloys, and clean surfaces of gold would thus be continually offered to the attack of the chlorine.
Hofman and Magnuson, however, have shown that there is a limit to this action, if it exists. They found that with pure gold and quartz grains an excess of chlorine in a rotating bottle dissolved pure gold a little faster than gold containing 10 per cent, of silver. The alloy gold 80, silver 20 was almost as readily dissolved by a supersaturated solution of chlorine as pure gold, but the alloy gold 70, silver 30 was acted on much less rapidly. The solvent power of somewhat weaker solutions was far more seriously interfered with by the presence of 20 per cent, of silver in the alloy.
In charging-in, the water is added first, being run in by a hose through the manhole, until it reaches a certain mark on the inside of the barrel. The amount of water to be used varies with the nature of the ore—roasted pyrites absorbing much more water than siliceous ores ; the quantity required is usually from 40 to 60 per cent, of the weight of the ore—i.e., from 80 to 120 gallons of water per 2,000 lbs. of ore. This is enough to make an easily flowing pulp. It is much more than that employed in the Plattner process, where it is necessary to keep the mass porous, so as to enable the gas to pass through it. In the barrel, however, this reason for limiting the amount of water does not exist, and the ore is really treated by a solution of chlorine water. If too small a quantity of water is used, so that the pulp is not quite free-flowing, lumps are formed which are not broken by the revolution of the barrel, and these lumps are not perfectly chlorinated.
The ore is let fall into the barrel down a shoot from an overhead hopper, which may conveniently be made to contain the exact quantity of ore required for a barrel charge. The ore should be perfectly dry (not cooled after roasting by too much “ wetting down ”), as otherwise it sticks in the hopper, instead of sliding freely down the shoot. The latter may be conveniently made of canvas, so that it can be looped-up out of the way when not in use. The chemicals (bleaching powder and sulphuric acid) may be added in one of two ways. Either the lime is thrown into the water before the ore is added, and the acid subsequently poured upon the upper dry surface of the latter, just before the manhole is closed or the acid is poured into the water, through which it sinks to the bottom without mixing, the ore let fall next, and the lime added on the top. This latter system is perhaps the better, as, if it is used, the generation of chlorine is never begun before the barrel commences to revolve, provided ordinary care is taken.
The amounts of bleaching powder and sulphuric acid to be added depend on the nature of the ore, and the care with which it has been roasted. If there is nothing in the ore which is open to attack except gold, the amount of chlorine actually absorbed in the course of the chemical reactions is extremely small, 1 oz. of gold requiring only 0.54 oz. of chlorine to convert it into the soluble trichloride.
The bleaching powder used should be of the finest quality obtainable, to prevent the introduction of an unnecessarily large amount of sulphate of lime into the charge. Bleaching powder usually has assigned to it the formula Ca(OCl)Cl, or Ca(OCl)2 + CaCl2, and may be, as the latter formula would indicate, a mixture of hypochlorite and chloride of lime. The reaction with acid is usually expressed thus:
CaCl2 + Ca(OCl)2 + 2H2SO4 = 2CaSO4 + 2H2O + 2Cl2
This equation certainly does not accurately represent what happens, as much less chlorine is liberated than is indicated by it. The amount of “available” chlorine (i.e., that which is liberated by the action of acids) contained in commercial bleaching powder varies from 20 to 35 per cent. Bleaching powder is gradually decomposed by the carbonic anhydride in atmospheric air (chlorine being liberated), and must consequently be kept separated from it as completely as possible. Even if preserved in hermetically sealed vessels, however, it suffers a slow change, by which some chlorate of lime is formed and the amount of available chlorine reduced. Under these circumstances, it is necessary to re-determine the value of the bleaching powder at short intervals of a few days. The shortest and best method of effecting this is to grind a sample in a stoneware mortar under water, and add the emulsion to an excess of a solution of potassium iodide. The whole of the available chlorine instantly displaces an equivalent quantity of iodine, which is set free and may be readily estimated by a standard solution of hyposulphite (thiosulphate) of soda in the usual manner. From this the amount of available chlorine in the sample is calculated. The whole operation can be performed in from ten to fifteen minutes when the standard solution has been prepared.
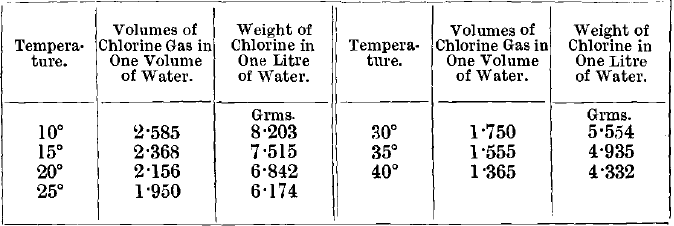
The amount of chlorine added to the barrel must be enough to yield a considerable excess at the end of the operation. It is usually considered that this excess should be enough to make the solution smell strongly of the gas when exposed to the air at the ordinary temperature and pressure. This is nearly equivalent to stating that at the end a saturated solution of chlorine in water should remain. If the water used is 100 gallons per ton of ore, the minimum amount of chlorine to be used should be, on this supposition, from 6 to 8 lbs. per ton of ore. This quantity may be taken as one well adapted for the efficient chlorination of ordinary ores. Nevertheless, much smaller quantities have been used with complete success on certain ores, and, as has been already stated, no general rule, applicable to all cases, can be laid down. Thus certain ores from Dakota, containing only 1 or 2 per cent, of sulphur, and consisting chiefly of silica, were chlorinated by the author in revolving barrels, using only 3½ pounds of chlorine per 2,000 pounds of ore. Even in this case, there was a strong excess of chlorine in the ore after the solution was complete, and the amount used could probably have been still further reduced without lowering the percentage extraction of gold. This ore contained 10 dwts. of gold to the ton, and over 80 per cent, was extracted. Most of the gold in the residues was locked up in coarse particles of ore. This was an extreme case, and it is seldom that so little chlorine is sufficient. When much oxide of copper or other metallic oxides capable of absorbing chlorine are present, or if the ore is insufficiently roasted, the pressure rapidly falls off, and a further addition of chemicals may become necessary, as at the Phoenix Mine. The total pressure exercised by the air and chlorine is sometimes made equal to two atmospheres—i.e., one atmosphere in excess of the normal—and this may be taken as the greatest pressure which it is advisable to maintain inside a barrel. If greater pressures are used, either very expensive barrels are required, or else the valves, manhole, &c., soon begin to leak.
It must not be forgotten that, at higher temperatures, water dissolves less chlorine than the amount mentioned in the last paragraph, and, consequently, a pressure of chlorine equal to that of the atmosphere will be obtained with the use of smaller quantities of chemicals. Thus at 40° C., with the other conditions identical with those given above, the minimum amount of chlorine required to yield a saturated solution at the end of treatment is only about 4.3 lbs., and, as the temperature rises above this, the quantity of chlorine necessary falls off rapidly.
For the amount of chlorine dissolved by water at different temperatures, see Schonfeld, Ann. Chem. Pharm., vol. xciii., p. 25 ; vol. xcvi., p. 8. The results are quoted in Roscoe & Schorlemmer’s Treatise on Chemistry, vol. i., p. 123, 1881. The following results, giving the amounts of chlorine contained in saturated solutions, have been abstracted, the volumes of chlorine being at 0° and 760 mm:
The temperature of the barrel is raised by several degrees by the chemical action. The exact amount in practice has not been determined. The relative amounts of bleaching powder and acid used is varied with different ores. Theoretically, according to the equation given above, 7 parts of chloride of lime require 6 parts of sulphuric acid for complete decomposition, but practically a little more sulphuric acid is always added, because it is desirable to maintain an excess of the acid in the charge. This is done in order to prevent lead and lime from getting into solution as chlorides, which would entail a loss of chlorine, and also to assist in dissolving any oxide of copper that may be present, which otherwise would also absorb chlorine. The proportions added are usually 1 part of bleaching powder to 1½ or 2 parts of sulphuric acid of 66° B.
The time occupied in chlorinating usually varies from three to six hours. The continued presence of an excess of chlorine gas should be tested from time to time by opening a small valve momentarily. If the presence of the free gas cannot be detected, the barrel must be opened and further supplies of chemicals added. The amount of pressure in the barrel is not alone sufficient to prove the presence or absence of an excess of chlorine, as other gases may be generated and exert considerable pressure. When the chlorination is finished, the excess of chlorine is discharged by a hose-pipe outside the building, the barrel is filled up with water, again revolved, and the liquid decanted on to large, shallow filter-beds. The barrel is again filled up, revolved, and decanted as before, and, finally, the whole charge is emptied out and another wash-water given to the charge on the filter. In comparing this method of washing by decantation with that of direct filtration, usually adopted, A. Thies found that, with similar charges, the amount of water used in the latter case was nearly double, and the time occupied much longer, while the tailings contained 5½ dwts. of gold per ton against about 1 dwt. when washed by decantation. He also found that there is little difficulty in filtering through a bed of fine ore from 3 to 4½ inches thick, but if the thickness of the bed is greater, direct leaching becomes very tedious and ineffective, and decantation is much better.
The leaching vats are usually constructed of wood, which is either lined with lead or coated with tar and pitch. They are usually round, but at Bunker Hill they were rectangular, measuring 6 feet by 18 feet, and 18 inches deep. They are lined with lead, and incline towards the drain hole, where the bottom is 1 inch lower than at the other side of the tank. The filter-bed was made of quartz-pebbles, gravel, and fine sand.
Mechanical Difficulties of Leaching
The difficulties of leaching vary enormously with the character of the ore and the treatment to which it has been subjected. Concentrates are among the best leaching ores, as, even if they were originally in a state of extremely fine division, the oxidising roasting in many cases appears to cause an agglomeration of the particles into porous granules, which do not pack down readily, and do not resist the passage of liquids. A small quantity of red oxide of iron in a very fine state of division is often present in roasted concentrates, and this is carried away by the water, passing into and partly through the filter-bed and even appearing in the precipitating vat. This material, on settling to the bottom of the gold solution, often appears to carry down with it some of the gold, forming a layer of rich slimes. In some cases, these iron oxide slimes are present in roasted pyrites in such quantites that leaching is greatly interfered with, and made very tedious. Siliceous ores, if properly pulverised usually present no difficulty in leaching, but aluminous ores are exceedingly troublesome. At Mount Morgan, Queensland, the ore formerly consisted chiefly of hydrated oxides of iron, which offered the greatest possible resistance to leaching if treated raw, but, if roasted, the loss of their water of hydration was found to be accompanied by a remarkable agglomeration of the particles of ore, and ordinary gravitation leaching was thus rendered possible.
In order to quicken the process of leaching various appliances have been suggested. Different forms of vacuum pumps have been used, the amount of air in the space below the filter-bed being reduced by them, and the liquid thus forced through by atmospheric pressure. In 1889, at the Colorado Gold and Silver Extraction Company’s mill at Denver, the effect of the use of increased pressure of air applied directly on the surface of the liquid was tried. A special cast-iron vat was constructed capable of sustaining an internal pressure of 100 lbs. per square inch, and furnished with valves, so that air and water could be simultaneously pumped into it. It was found that ores, which entirely prevented the passage of water through them, even after a vacuum of 20 inches of mercury had been established beneath the filter-bed, could be leached with great speed under a pressure of from 30 to 50 lbs. per square inch. Moreover, when the leaching was complete, the ore could be freed from the water more completely by the passage of a current of air through it than by gravitation alone. This method, which was suggested by D. Dennes, the Company’s engineer, was modified by J. E. Rothwell, who placed the filter-bed inside the barrel. The chief objection to it seems to lie in the additional expense incurred in the construction of the barrels or leaching vats and in working the pumps.
Mr. Riotte long ago suggested that the wash-water should be thoroughly mixed with the ore by agitation, and then removed as completely as possible by squeezing in a screw filter-press. He used a pressure of from 25 to 30 lbs. per square inch, and found that the average amount of moisture retained in the ore after being squeezed was about 6 per cent. As this would cause the retention in the tailings of from 3 to 6 per cent. of the soluble gold, according to the amount of wash-water used, the method is obviously inapplicable to rich ores, which would require to be subjected to treatment twice. Centrifugal leaching has also been proposed, and it is stated that the Mount Morgan ore can be leached in this way without previous roasting.
How Gold Precipitates after Chlorination
A number of reagents have been used or proposed for this purpose. An account of the chief methods is given below.
Ferrous Sulphate
This was used by Plattner and is still widely employed. The chief difficulties in connection with it are due to the slowness with which the precipitated gold settles and to the difficulty of collecting it by filtration. Some account of its use has already been given on p. 251. It is made by dissolving iron in sulphuric acid, with the aid of heat, and the crude solution thus prepared, which is in most cases added direct to the gold solution, always contains some free sulphuric acid. Precipitation takes place according to the equation:
2AuCl3 + 6FeSO4 = Au2 + Fe2Cl6 + 2Fe2(SO4)3
The oxidation of the ferrous salt is also effected in other ways,, notably by the excess of free chlorine present in the solution, so that much more sulphate of iron is required than is indicated by the equation. The difficulty of collecting and saving the precipitated gold has already been dwelt on. The gold settles better if it is well stirred, and Aaron recommends an addition of more sulphuric acid and vigorous stirring, two hours after the precipitation is complete, as a means of assisting the settling. Besides gold, the only other metals precipitated by ferrous sulphate are those which form insoluble sulphates—viz., lead, calcium, strontium, and barium. The last two of these are rarely present, and the others are dealt with in the manner already described above. Basic iron salts are not precipitated if enough free sulphuric acid is present, and when precipitated, they may be removed from the gold by treatment with acids, or by slagging them off in the furnace.
Other proto-salts of iron are equally efficacious, and ferrous chloride has been used in the aqua regia parting process.
Sulphuretted Hydrogen
This was formerly made at Deloro by heating paraffin and sulphur together, and the gas diluted with air was forced through the solution by means of a small air-pump. The use of the air was to keep the solution agitated, and to expel part of the chlorine mechanically, and so economise the sulphuretted hydrogen, which is decomposed by chlorine. The equation for this decomposition is given by Langguth as follows:
H2S + 4H2O + 8Cl = H2SO4 + 8HCl
but it is more likely that the greater part of the change is represented by the well-known equation:
H2S + Cl2 = 2HCl + S
although a small amount of sulphuric acid may be formed at the same time. The precipitate of sulphur thus formed before and during the precipitation of the gold, which begins before the whole of the chlorine has been destroyed, is to be avoided, and Langguth has, therefore, suggested the use of sulphur dioxide, generated by burning sulphur to destroy the free chlorine, the reaction being as follows:
Cl2 + SO2 + 2H2O = H2SO4 + 2HCl
When almost all the chlorine has been thus converted into hydrochloric acid, the point being determined by testing some of the liquid with a few bubbles of sulphuretted hydrogen, the passage of SO2 is stopped, and sulphuretted hydrogen, now generated by the action of sulphuric acid on iron matte, is forced into the solution, destroying the last traces of chlorine and precipitating the gold. This system was introduced at the Golden Reward Chlorination Works in 1891, and was subsequently adopted at other mills in the United States, but the use of SO2 was afterwards abandoned.
The gold is precipitated as a sulphide mixed with more or less sulphur, the reaction being represented by the following equation:
2AuCl3 + 3H2S = Au2S3 + 6HCl
It is said to take less than an hour at the Golden Reward Works to precipitate the gold from 5,000 gallons of solution (resulting from the lixiviation of from 25 to 50 tons of ore). The liquid is quite cold, but the precipitate is in a collected, voluminous and flocculent form which settles quickly. It is left undisturbed for two hours, and the liquid is then drawn off to within 4 inches of the bottom of the vat, and passed through a filter-press, provided with a set of heavy, canton-flannel filter- cloths. The head of liquid used for filtering is 25 feet, and the filtration is said to occupy from three to four hours, according to the amount of sulphides already contained in the filter. When the latter is full, a small air-pump is connected with it and a current of air passed through it for an hour to dry the mass of sulphides into hard cakes, which are easily handled and removed. The precipitate is then roasted in iron trays, which are placed in cast-iron muffles and heated only from the top, no stirring being necessary, so that the loss from dusting is small. The filter-cloths are burnt with the precipitate, when they have become either clogged with sulphides, or untrustworthy owing to the action of the acid liquors. The precipitate is then melted down with a little borax and nitre, the total loss in handling being very small. The bullion is about 900 to 950 fine in gold, the remainder consisting chiefly of silver, copper, lead, and arsenic. The bulk of the precipitate remains at the bottom of the vat. It is allowed to accumulate for a fortnight, and is then filtered and treated as above. The slag resulting from the fusion of the gold is crushed and melted down with litharge, and the reduced lead cupelled (Rothwell).
It has usually been assumed that all the lead, copper, and silver contained in the liquids are precipitated with the gold, and that if much copper is present, the bullion will be very base, and Langguth suggested the removal of the copper from the precipitate by dilute nitric acid. Rothwell, however, has stated (Mineral Industry for 1896, p. 278) that, under normal conditions, the gold can be precipitated, forming a bullion from 820 to 960 fine, whilst the copper remains in solution together with barely a trace of gold.
Sulphurous Acid
Sulphurous Acid, which has been already mentioned as a cheap agent for destroying free chlorine, precipitates gold very completely from dilute solutions, but its action in the cold is slow until the liquid is almost saturated with the gas (water absorbs 39 volumes of the gas at 20°), and the gold settles slowly. At higher temperatures the action of the gas is much more rapid and satisfactory, and very little is wasted in saturating the liquid. As a precipitant, the gas does not appear to present any advantages over ferrous sulphate.
Charcoal
Henry referred to the reducing action of charcoal on chloride of gold in solution, and observed that the gold will be precipitated on the charcoal, if the solution is either exposed to the direct light of the sun or heated to 212° F. In 1869, in Percy’s laboratory, some sticks of wood-charcoal were immersed in water, and 32.50 grains of gold in the form of chloride were added on August 7. 85 more grains of gold, in the form of chloride, were added on November 3, 1869, and the bottle was left to stand. This bottle is in the Percy collection, at South Kensington, and the surface of the charcoal is now coated over with metallic gold which shows on its surface the fibres and vessels of the stem.
Vegetable charcoal was adopted as a precipitant at the Mount Morgan Mine, Queensland, in 1887. The method adopted there is as follows:—The solution is heated, the free chlorine being thus expelled ; the liquid is then made to run slowly through large shallow tanks, each 10 feet by 11 feet and 4 feet 6 inches deep (see Fig. 65). They are built of brick on a concrete foundation, and lined with Portland cement and tar. The layer of charcoal in them is 2 feet deep, rests on a filter-bottom, and is covered by thick perforated sheets of lead. The liquor flows downwards through three charcoal filters in succession, 660 cubic feet of charcoal being sufficient for the treatment of the liquor from about 100 tons per day of 11 dwts. ore. In preparing the charcoal for the filters, it is crushed, and all that passes a 20-mesh and remains on a 30-mesh screen is called “coarse,’’ whilst that which passes the 30-mesh and remains on a 40-mesh screen is called “ fine.” 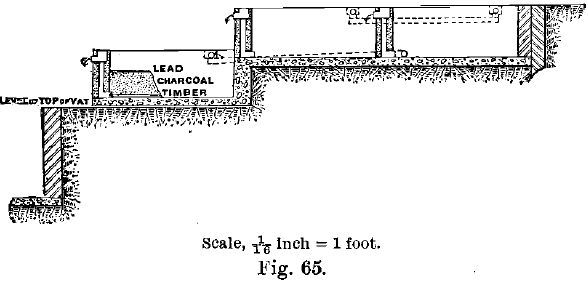 All that passes 40-mesh is thrown away. Two cubic feet of fine are used to 1 cubic foot of coarse, the coarse being at the top and bottom. The charcoal is well rammed in with the foot, especially round the walls. For seven months the liquor running away from these filters assayed in one mill 0.76 grain gold per ton, and in the other 1.89 grains, per ton. After passing the charcoal, all liquors are run through a concrete tank with a bed of sawdust 1 foot thick and more gold caught in this way. When the charcoal is coated sufficiently with gold, it is burnt in small furnaces furnished with dust chambers. The ashes were formerly amalgamated, and the rich slimes sold to the smelters. Afterwards the ashes were fluxed in crucibles and eventually in a small reverberatory furnace, the fluxes used being soda ash and borax, which were mixed with the ashes and then moistened with water. D. Clark states that the charcoal ash contains from 20 to 40 per cent, of gold. The slag contained from 20 to 30 ozs. per ton. Animal charcoal cannot be used owing to the difficulty of burning it afterwards.
All that passes 40-mesh is thrown away. Two cubic feet of fine are used to 1 cubic foot of coarse, the coarse being at the top and bottom. The charcoal is well rammed in with the foot, especially round the walls. For seven months the liquor running away from these filters assayed in one mill 0.76 grain gold per ton, and in the other 1.89 grains, per ton. After passing the charcoal, all liquors are run through a concrete tank with a bed of sawdust 1 foot thick and more gold caught in this way. When the charcoal is coated sufficiently with gold, it is burnt in small furnaces furnished with dust chambers. The ashes were formerly amalgamated, and the rich slimes sold to the smelters. Afterwards the ashes were fluxed in crucibles and eventually in a small reverberatory furnace, the fluxes used being soda ash and borax, which were mixed with the ashes and then moistened with water. D. Clark states that the charcoal ash contains from 20 to 40 per cent, of gold. The slag contained from 20 to 30 ozs. per ton. Animal charcoal cannot be used owing to the difficulty of burning it afterwards.
The exact action of the charcoal has not been fully demonstrated. It acts slowly on cold solutions, and its action is not rapid even at boiling point. It is under the disadvantage that it does not destroy free chlorine, which must therefore be expelled by boiling or by passing a current of air through the liquid before the precipitation of the gold is begun. Mr. Davis states that 240 parts of charcoal are required for the precipitation of 19¼ parts of gold. The prevailing opinion is that the hydrogen and hydrocarbons remaining in the charcoal are the active agents in the precipitation, hydrochloric acid and free gold being formed.
Copper Sulphide and Iron Sulphide
Copper Sulphide and Iron Sulphide, either precipitated or fused, have been proposed, but apparently not used for any length of time in practice. The sulphides are not mixed with the solution of gold chloride, but contained in filter-boxes, through which the solution is passed. The free chlorine is destroyed by the sulphides, and the iron or copper gradually removed. Iron or Copper Shavings were also proposed by the author as a precipitant for gold, but have not been used.
Electrical Precipitation
It is stated that this was tried by W. Greenawalt, who used over again in the barrel the solution from which the gold had been precipitated. In this case the imperfection of the precipitation was of little moment, but in general solutions become too highly charged with salts of lime and base metals to be conveniently used more than once.
How Barrel Chlorination Works
In 1890, improved methods were introduced by J. E. Rothwell at the Golden Reward Mill, S. Dakota, for treating ores not concentrates, and mills were erected later at Cripple Creek and other places in Colorado and Dakota. Further slight improvements were made from time to time, and in 1896 Rothwell stated that 1,000 tons of ore a day were being treated by barrel chlorination in America, but the cyanide process is now generally preferred, and the industry seems to be languishing.
The ores are crushed dry in rock-breakers, followed by two or three sets of rolls, the finished product passing through 12- to 20-mesh screens. The ore is dried before being finely crushed. The roasted and cooled ore is then charged into large barrels, which had a capacity of 3½ tons at the Golden Reward, but hold as much as 12½ or even 18 tons in later practice. The 12½-ton barrels are 16 feet long inside and 5 feet 6 inches in diameter. The trunnions are 16 inches in diameter. The barrels consist of a steel shell of 5/8-inch thickness, lined with ½-inch pure lead of 24 lbs. to the square foot. The lining is bolted to the shell. There are two oval charging-holes, each 11 x 16 inches. The cast-iron heads are 1¾ inches thick.
An enlargement of part of the end of a good type of chlorination barrel is shown in A, Fig. 66; in B, the construction of Rothwell’s barrel is shown. The shaded parts of A and B are made of iron (the barrel-head and the flange being cast iron, and the cylinder, boiler-steel); the lead lining is not shaded. In A the lead lining is burnt-on to the flanged cylinder and to the head respectively, and the head then firmly bolted to the flange. The lead-joint, C, is made tight by the blows of a hammer on a blunt chisel directed on the surface of the lead, in the direction shown by the arrow. When repairs are needed inside the barrel, the cast-iron head is removed and the work thus easily done. In the other barrel the lead lining of the end is burnt-on to the lining of the cylinder at D, leaving a hollow space, E. It is stated by Dennes that, in consequence of the existence of this space, the lead-joint is often broken when the pressure in the barrel is great, and leakage then occurs; moreover, repairs must be effected by a workman crouching inside the barrel.
The chlorination barrel is also the washing and leaching vessel; this is arranged by placing a supporting diaphragm, for a filtering medium, to form the chord of an arc of the circle of the barrel. The filter or diaphragm, as it is called, was at first made of asbestos cloth, resting on a framework consisting of oaken planks, each 11 inches wide and as long as the barrel, and 2 inches thick. The area of the filter in the 12½-ton barrel is 4 by 16 feet, or 64 square feet. The planks were grooved, and the filter-cloth rested on the sharp ridges between the grooves, the surface being almost entirely available for filtration. Above the asbestos cloth was placed an open wooden grating, and the whole was held in place by cross-pieces, the ends of which rested under straps bolted to the inside of the shell. The cloth lasted for from 15 to 18 charges, or from 2½- to 3 days. In later practice the asbestos cloth was replaced by 4 lbs. sheet-lead, with fine perforations, supported by sheet-lead 3/8-inch thick with 3/8-inch perforations, and enclosed between wooden gratings. The thin sheet-lead lasts from 6 to 60 charges. A sand filter has also been used inside the barrel.
The barrel is charged by first filling the space under the filter with water, which at the same time is allowed to pass through the filtering medium, and wash it; then the required quantity of water is put in above the filter. The sulphuric acid is then poured into the water, through which it sinks in a mass to the bottom, without mixing with it; the ore is then charged in, as follows :—The hoppers are furnished with 2 shoots, one for each charging-hole. The ore is let fall through these shoots alternately, the hole through which ore is not being passed serving as an air-vent. Meanwhile the bleaching powder has been weighed- out and placed in two small kegs. When the ore has all been introduced into the barrel, a workman, stationed at each charging-hole, hollows out a space in the surface of the dry ore with his hands, and, emptying one of the kegs into the barrel, closes up the charging-hole as quickly as possible. If all these operations have been conducted rapidly without a hitch, there is no immediate evolution of chlorine, but, if some time is suffered to elapse after charging-in the ore, the acid liquid, thoroughly stirred-up and mixed by the fall of the ore into it, gradually rises through and wets the charge, and the bleaching powder, falling on ore which has been wetted with acid, gives off copious fumes of chlorine before the cover-plate can be screwed on.
After the chlorination is complete the barrel is stopped, so that the filter assumes a horizontal position ; the hose is attached to one of the outlet pipes, and after waiting for a few minutes to allow the pulp to settle, as recommended by Rothwell, the valve is opened and the solution allowed to run out, the pressure of gas left in the barrel being enough to start the leaching. A hose is also attached to the inlet pipe, and water is pumped in under a pressure seldom exceeding 40 lbs. per square inch, the air in the top part of the barrel being compressed and forming an elastic cushion. By washing in this manner, no chlorine is allowed to escape into the building, as it is all absorbed by the water. If necessary the leaching is suspended at intervals, and the barrel is again revolved for a few minutes, so that its contents are thoroughly mixed-up together again. In this way the formation of permanent channels in the ore is prevented, and perfect leaching is ensured. The wash-water coming from the barrel is tested for gold with sulphuretted hydrogen. The full charge of ore is said by Rothwell to lie to a depth of 38 inches on the filter in his 12½-ton barrel, and the average time of leaching to be 2½ hours. The amount of water required for leaching is about 120 gallons per ton, besides the 100 gallons per ton contained in the charge.
The tailings are discharged into a car which will hold the whole charge of ore and water, and then run out of the building; or, if water is abundant, they are discharged into a sluice, and washed away. The filter-cloth is washed clean by a jet of water under pressure directed successively to all parts of it. This water is discharged by revolving the barrel.
The solution coming from the barrel, passes first to a tank with a gravel filter and then to the settling tanks, which are of sufficient capacity to allow several hours for settling. The clear liquid from these is forced up by air pressure into the precipitating vats.
At the Golden Reward Mill there were two precipitating tanks for each barrel. They were 6½ feet in diameter and 10½ feet deep, fitted with covers, and were constructed of wood and lined with lead of 8 lbs. to the square foot. The precipitated sulphides were collected in a filter press, situated on the ground-floor, over 25 feet below the precipitating vats. The press had twelve chambers of 19 inches, and had a filtering area of 57 square feet. Two lead pipes connected the precipitating tanks with the press, one leading from the bottom of the tank and the other from a point in the side about 4 inches above the bottom. The precipitating gas generators were cast-iron cylinders of the same size as the slime filters ; only the sulphuretted hydrogen generator was lined with lead.
It is stated that solutions containing gold, copper, arsenic, antimony, &c., yield a precipitate containing little but sulphide of gold, if precipitation is stopped at the right moment, and if free acid is present. The sulphide cakes in the presses are compressed by blowing air through them, and are turned out in the form of lumps, which break up into powder when they are touched; they are then dried, roasted in muffle furnaces in iron trays, 44 inches long, 24 inches wide, and 4 inches deep. The roasted precipitate, which may contain as much as 75 to 80 per cent, of gold, is fused with borax, nitre, carbonate of soda and sand.
Bromination
The following details were supplied to the author by D. Dennes. The ore which was treated in 1892 contained 6 or 7 per cent, of sulphur, a similar amount of arsenic, and some iron, but little or no copper. It was roasted in White-Howell and Bruckner furnaces, and charged into lead-lined revolving barrels, 8 feet long and 4½ feet in diameter, and having a capacity of 3½ tons. Hot water was introduced into the barrel before charging-in the ore, only 66 gallons per ton or 33 per cent, of the weight of the ore being used. This seems too small a quantity for efficient treatment. The barrels had cast-iron trunnions 12 inches in diameter, and were revolved at the rate of twelve turns per minute by a worm and wheel. They were made of boiler iron, 3/8 inch thick, and were lined with thick lead of 24 lbs. to the square foot on the ends, and 18 lbs. to the foot on the cylinder.
After adding the ore and water, the required amount of bromine was poured in, and the barrel closed at once. Little inconvenience is stated to have been caused to the workmen by the bromine fumes which were evolved owing to this crude method of manipulation; the men’s eyes only were slightly affected. From 6 to 13 lbs. of bromine were added to the charge of 4 tons, the amount being adjusted so that a very slight excess of free bromine remained at the end of the operation. Chlorine generated from sulphuric acid, and bleaching powder was used for several months before the introduction of bromine, but the cost was greater and the tailings only ¼ dwt. poorer in the former case. Moreover, the filter cloths lasted longer, the health of the workmen suffered less injury, and the gold precipitate was purer when bromine was used. The barrel was revolved for from thirty minutes to one and a-half hours, and then discharged into the leaching vat below. This was 7½ feet in diameter and 3 feet deep, made of cast iron lined with lead, and had a strongly ribbed cover, so that it was capable of withstanding an internal pressure of 100 lbs. to the square inch. Internally, it tapered slightly upwards. The sides of the vat did not rest on the bottom, but were supported by columns direct from the floor. The bottom was not connected with the rest, but was supported by a hydraulic ram. On this true bottom there was a filter-bed and false bottom. The filter-cloth consisted of gunny-sacking. The vat was worked as follows When a charge was about to be introduced, the bottom was raised by the ram and pressed tightly against the sides of the vat, a good joint being made by a thick rubber ring. The hydraulic pressure was more than enough to overcome the air pressure subsequently applied. The charge was then introduced, the cover replaced and fastened down tightly by screw-bolts, and air was pumped into the vat above the surface of the charge. Water was also introduced by means of a lead pipe extended round the vat at about the surface of the charge. This pipe was pierced with a number of small holes, by means of which jets of water were thrown upon all parts of the ore surface. The air pressure, which never exceeded 60 lbs. to the square inch, forced the wash-water through the charge very quickly. The solution was of a strong ruby-red colour, due to the presence of free bromine, and of the bromide of gold, the colour of which in solution is very intense. There was no need to test the issuing liquid, as its colour was found to be a perfect guide to an experienced eye. When it had become colourless, the water supply was shut off and the charge dried by pumping air through it. This was necessary, as the company was not allowed to sluice the tailings into the river. When no more water could be driven out, the air-pressure was let off, and the bottom of the vat lowered until the top of the filter-bed was just clear of the sides of the vat. The bottom was then drawn sideways from below the vat by means of a second hydraulic ram, and the charge fell into a large ore-car below, having a capacity of 4 tons. This ore-car then ran by gravitation to the dump, and was emptied and drawn back by a wire rope winding on a drum actuated by steam power. It is stated that the leaching occupied only twenty minutes, and the vat was used to leach the charge from the two barrels, which discharged into it alternately. The vat was so rapid that it was always waiting for the barrels, and 100 tons per day could be leached by it.
The liquid was forced into large, lead-lined wooden precipitation vats, each 20 feet x 10 feet x 6½ feet, which were placed outside the mill, buried under 2 feet of manure to keep them warm in winter. The precipitation was effected by the successive use of sulphur dioxide and sulphuretted hydrogen, applied in exactly the same manner as at the Golden Reward Mill. The collection of the sulphides was also effected in a similar manner. When dried, the sulphides were put, together with the necessary amount of borax, into a small barrel, revolving by machinery, and were mixed thoroughly. The mixture was then transferred by a scoop to a red-hot clay crucible (size No. 100) in the furnace, additions being made at intervals until the crucible was full of bullion and molten slag. The latter was very rich and full of shots of metal. It was stored and eventually sold to the smelters, but owing to the difficulty in sampling it, it would have been better to fuse it with lead and to sell the latter.
The air-compressor used was 12 inches in diameter, and had an 18-inch stroke; its maximum velocity was eighty revolutions per minute. A large air receiver, 16 feet long and 4 feet in diameter, was used between the pump and the leaching vat. The maximum air pressure in the receiver was 80 lbs. per square inch. From 60 to 75 per cent, of the gold in the ore was extracted, the tailings usually containing over 5 dwts. of gold. The reason for this unsatisfactory result is said by Dr. L. D. Godshall (Eng. and Mng. Journ., Jan. 6, 1894) to lie in the coarseness of the crushing, a 10-mesh sieve being used. When a 30-mesh sieve was used, only about 2 1/8 dwts. were left in the tailings, and the product was easily leached. It seems possible that inefficient leaching, due to the formation of channels in the ore, may have been another cause of loss, but the chief cause of the failure was doubtless the fact that the amount of bromine used was inadequate, the endeavour to reduce the costs of the process being fatal to success.
