Bacterial Leaching of Copper Ores
Several types of bacteria capable of living in an acid environment have been isolated from copper mine waters. The foremost of these are of the genus Thiobacillus (T.). Thiobacillus bacteria are acidophilic aerobic chemolithotrophs which grow most rapidly at a pH in the range of 2-3. T. ferrooxidans derive their energy by oxidizing ferrous iron, elemental sulfur and reduced sulfur compounds and utilize carbon from carbon dioxide for biosynthesis (Hutchins et al, 1982). T. thiooxidans oxidize only reduced sulfur and can assist in attacking copper sulfides. The ability of T. ferrooxidans to adapt to differing concentrations of metallic ions has been documented in published research.
Several other bacteria have been found to oxidize iron and/or sulfur under various pH and temperature conditions. Among these are Leptospirillum ferrooxidans, Sulfobacillus thermosulfidooxidans, and genus Sulfolobus; and no doubt, there are many others yet to be isolated or discovered. The Sulfolobus are the most promising of the thermophilic acidophilic microbes, but they have been studied much less than the T. ferrooxidans.
The optimum ore particle size, nutrient composition, bacteria, ore and ferrous iron concentrations, as well as pH and temperature have been experimentally established for leaching with T. ferrooxidans bacteria. Several successful laboratory leaching experiments and field tests have been performed.
The biological role of the leaching reactions involves three mechanisms: indirect and direct leaching and galvanic conversion. The term indirect leaching is used here to describe the role of T. ferrooxidans in regenerating the ferric sulfate which dissolves the copper sulfides. Direct leaching occurs when the T. ferrooxidans or other bacteria directly attack the mineral surfaces. Galvanic conversion occurs when a galvanic cell is created between the electrolyte formed by the dilute sulfuric acid and Ferric sulfate solutions and two dissimilar metal sulfide phases which act as the anode and cathode.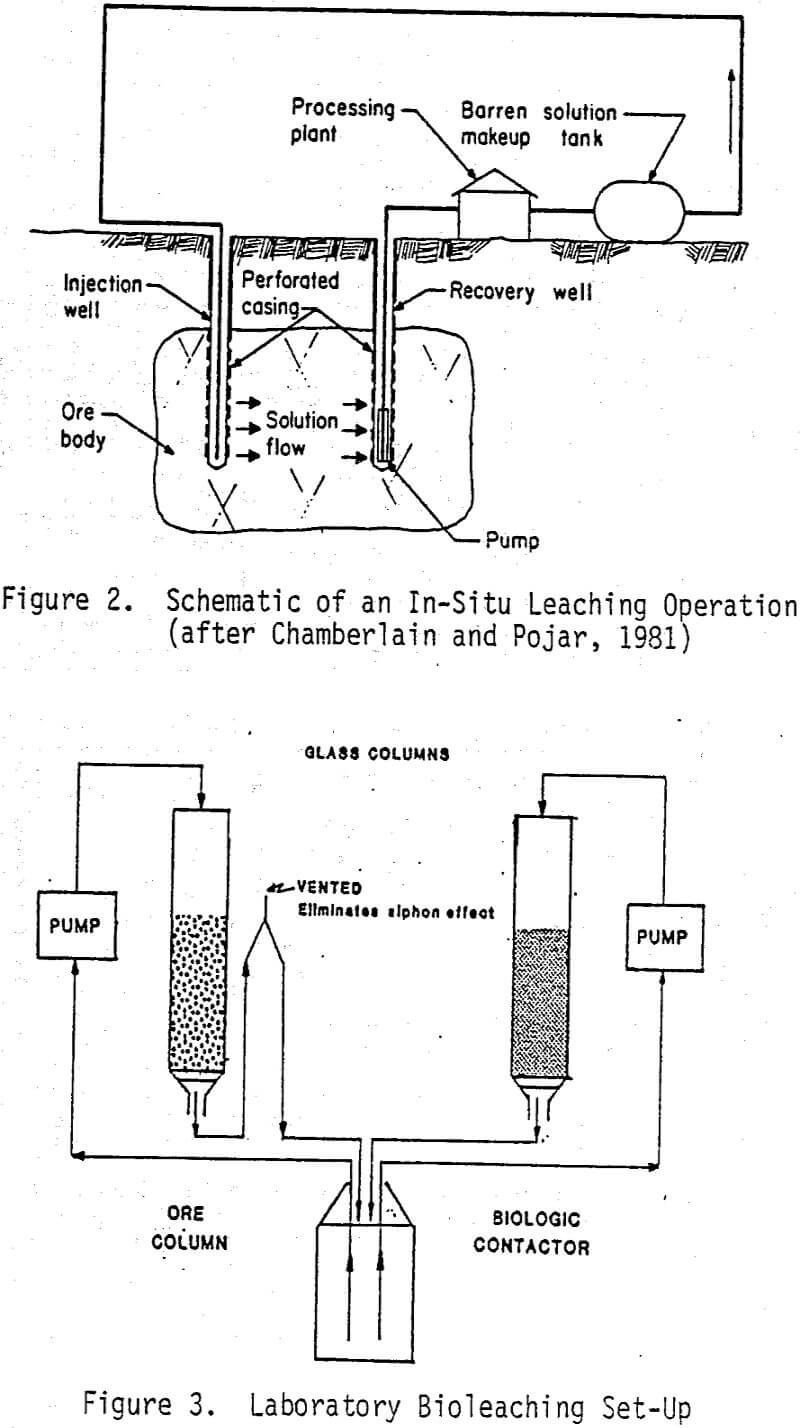
The contribution of indirect leaching has been studied the most while little is known about the contributions of direct leaching and of galvanic conversion. All three mechanisms are believed to operate in heap leaching situations. However, direct leaching by the bacteria will probably not be significant in in-situ operations because the low oxygen concentration and perhaps elevated pressure at the ore body depth will retard bacterial activity.
A commercial application of this bacterially-assisted leaching technology would involve injection of the ferric sulfate solution into the ore deposit where it dissolves the copper. The pregnant leach solution would then be pumped to the surface where the copper could be recovered. The solution would then contain ferrous sulfate as a result of the ferric sulfate oxidation of cuprous sulfide to cupric sulfide and of the sulfide ion to free sulfur or sulfate. The reduced ferrous sulfate product would then be oxidized back to ferric sulfate by simultaneous contact with oxygen and bacteria in a biological contactor and cycled back into the ore deposit. In this system bacteria would not be intentionally contacted with the copper sulfides and, therefore, little direct leaching would be expected to occur. The chemical reactions controlling copper dissolution and precipitation and those governing the biological regeneration of the ferric sulfate solutions are given in several papers. A schematic of an in-situ leaching operation is shown in Figure 2.
In conclusion, the study has revealed the presence of a copper reserve from which the copper, most of which is in the form of Cu2S, can be recovered by bacterially assisted ferric sulfate leaching and the economics look favorable at present. Research on Thiobacillus ferrooxidans bacteria indicates that strains are available that can be adapted to temperatures approaching those we would expect to exist in the leach solutions after they are passed through the underground copper deposit. It was found that certain strains could be adapted to copper in solutions at concentrations as high as 15 to 20 g/L. However, there was no evidence to suggest that the bacteria could use Cu+¹ as an energy source. Up to 80 to 90% of the copper was found to be recoverable from Bohemia type ore by ferric sulfate leaching in combination with bacterially-assisted ferric sulfate regeneration over a period of 75 to 80 days. In this same period about 70% of the copper was extracted from the single sample of Kona ore and about 60% from the single sample of White Pine ore.
The copper extractions were unaffected by a number of changes in the ferric sulfate regeneration apparatus (SBC) indicating that the capacity of the SBC used in our experiments was oversize with respect to the rate at which ferric iron was reduced. An intermediate-scale laboratory test is needed to determine the relationship between the capacity of the SBC and the size of the block of ore being leached at a given copper extraction rate and to investigate the effects of SBC variables on the copper extraction rate.
BACTERIAL OXIDATION OF A REFRACTORY ARSENOPYRITE GOLD CONCENTRATE
THERMOPHILIC HEAP LEACHING OF A CHALCOPYRITE CONCENTRATE
Thermophilic_Bioleaching_of_Chalcopyrite
Copper_heap-leaching-from_Chalcopyrite
Bioxidized-Sulfur_Formation_Samples Biooxidation Technology refractory gold deposit
Heap Leach-Laboratory-and-Demo-Scale-Operation-of-Caraiba-GEOCOAT

