How to make and use a blowpipe to test and perform a metal analysis for the presence of various minerals by evaluating the nature of the flames. Methods of testing in an open tube and a tube closed at one end.—On charcoal with carbonate of soda.—With borax and microcosmic salt on platinum wire.—Tables of reactions -with borax and microcosmic salt.—Testing with nitrate of cobalt.— General table (for the qualitative analysis of metallic substances).—Confirmatory tests.—To detect certain common substances associated with metáls.—Temporary blowpipe.
Apparatus required consists of the following
- Blowpipe
- Candle or lamp (fed with oil or melted tallow)
- Forceps with platinum points
- Charcoal
- Steel forceps
- Platinum wire and foil
- Magnet or magnetic needle or magnetic knife blade
- Knife
- Mortar (agate is the best material) and pestle
- Borax
- Microcosmic salt
- Carbonate of soda in small boxes.
In addition to the above, a small bottle of hydrochloric acid, and also some nitrate of cobalt solution, will be most useful. A few small open glass tubes, and glass tubes closed at one end. Many other articles might be of great use, such as a small aluminium plate, some nitric acid, sulphuric acid, zinc for confirmatory tests, and also hyposulphite of soda ; at the same time, they are not absolutely necessary.
In testing the quality of a mineral by the blowpipe, a small but well-chosen fragment about the size of a mustard- seed is sufficient.
In using the blowpipe, the principal thing to learn is to blow and breathe at the same time without removing the mouth from the instrument. This is effected by filling the mouth with air and gently blowing, and at the same time by breathing through the nostrils.
A lamp with a large wick, and fed with olive oil or melted tallow, affords a good flame, and so does an ordinary candle with a broad wick.
The blowpipe flame consists of two parts, the blue one (made up of inflammable gases) and the yellow one. To obtain the reducing flame the blowpipe jet should be just over the wick of the candle or lamp (Fig. 19). The specimen should be kept in the luminous part of the flame for some time.
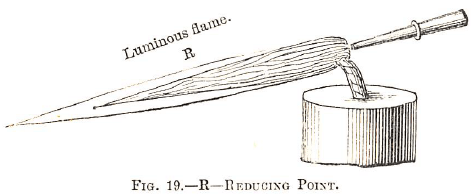
To obtain good results in this name is not always easy to a beginner, who however, will be more successful with the oxidizing flame. At or beyond the extremity of the yellow one (the whole of the gases being consumed
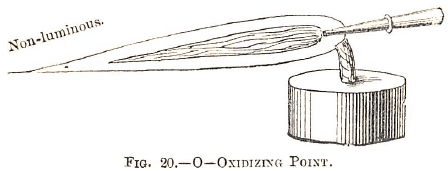
bodies are combined with oxygen, and this is called the “oxidizing” flame. To produce it properly, the blowpipe should be placed a little farther into the flame, and the operator should blow more strongly (Fig. 20).

Treatment in a tube closed at one end (Fig. 21) is best conducted over a spirit lamp. When the substance is to be heated in an open glass tube (Fig. 22), the tube should be inclined so as to allow a current of air to pass through (N.B. By heating a point of a straight tube in a spirit lamp, the tube may be bent into the required angle.) The charcoal on which the mineral is to be heated ought to be made from very light wood—such as elder, pine, &c.—and which, when heated, should be as free from smoke and ash as possible.
To treat the substance on charcoal, a small cavity should be bored on the edge of the grain in the top part of the charcoal by means of a knife-blade, and when the blowpipe flame is directed on the specimen, the support should he held in an inclined position, in order that the incrustation deposited on the cool portion can be properly noticed.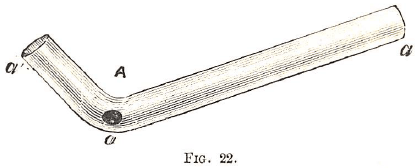
An aluminium plate about 4 inches long by 2 broad, and 1/32 inch thick, and with half an inch at the end bent nearly at right angles to the other part, and on which the specimen can be rested, is a capital support; only, as the plate is apt to become very hot during an operation, it must be held by tongs, the handles of which are wadded, so as not to come in contact with the operator. In using this support the specimen may be placed on a thin piece of charcoal. The incrustations on the aluminium plate are thicker than those on the charcoal support, and they can easily be experimented on by the blowpipe. When the operation is over, the plate may be cleaned by rubbing it with fine bone ash, by means of a piece of wash-leather.

Firstly, treat the substance alone on charcoal, and notice the effect of the oxidizing and then of the reducing flame on it. After which, treatment with carbonate of soda, and afterwards with borax and microcosmic salt, may be necessary.
As, sometimes, metals cannot be reduced from minerals by simply heating on charcoal alone, carbonate of soda is made use of. The substance should be very finely powdered and mixed with slightly moistened carbonate of soda, then placed in the cavity of the charcoal, and a gentle heat applied to it in order to drive off moisture ; afterwards the temperature should be considerably increased. Not only must the colour of the incrustation be noticed, but also the fused substance along with some of the charcoal ought to be removed, and ground up with a little water in an agate or porcelain mortar. More water should be added, and the whole mixed up ; the water, together with the lighter matter, should be poured off very carefully, which may be done with the help of a small glass rod or pencil placed at the side of the tilted-up mortar, so as to allow the water to run gradually down the side. The residue at the bottom of the mortar is thus ready for examination, and the metallic fragments, if any, will be seen by the naked eye or a magnifying- glass as glistening spangles or as powder.
When there is no incrustation, the metals—gold, silver, and copper—if present, yield glistening beads, and iron, nickel, cobalt, leave a magnetic grey powder.
Should there be an incrustation, the General Table C must be consulted, though each of the metals—silver, tin, lead, antimony—may be recognised in the residue by its characteristic appearance. As a rule, one ought not to rely upon the treatment with carbonate of soda, rather confirm by that with borax and microcosmic salt.
The usual fluxes, borax and microcosmic salt, readily dissolve metallic oxides at a high temperature. In order to make sure that the substance is in the state of oxide, it should be exposed to a gentle heat and roasted, in order to drive oft sulphur or arsenic associated with metals in the mineral.
To treat with either of these fluxes, bend the end of a small platinum wire round the point of a pencil into a loop of this shape, but smaller in size—

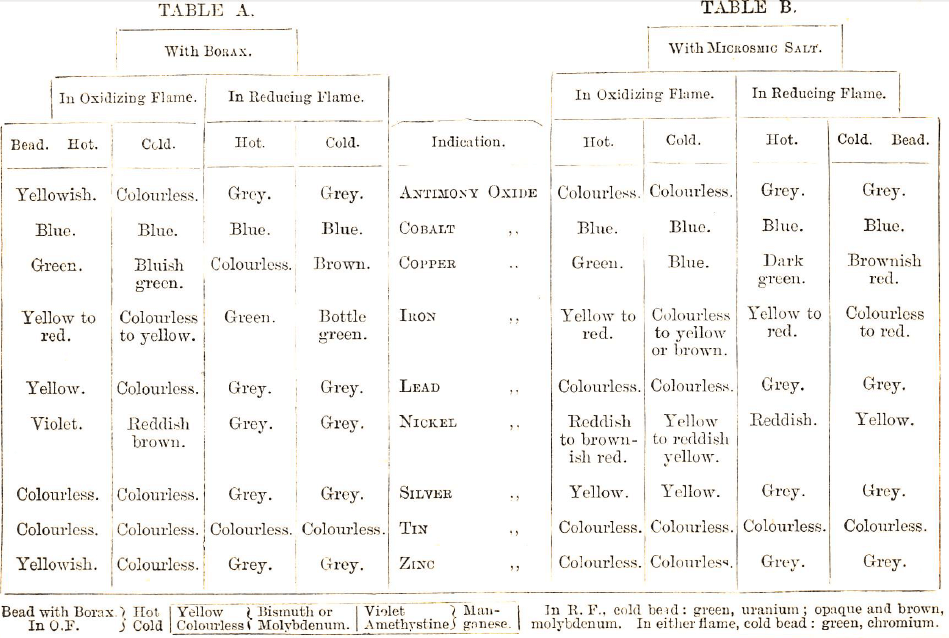
Moisten the loop, and dip it into either borax or microcosmic salt, and then heat it in the blowpipe flame till the flux is fused. When the head is soft or moist, it must be brought in contact with a very small quantity of the powdered mineral, and then exposed to the heat of the oxidizing flame, and afterwards to that of the reducing flame, the change of colour of the head when hot or cold, and the effect of each flame on it, being carefully observed.
If the substance, after heating, be moistened with nitrate of cobalt solution, and again strongly heated, it may when cool afford some clue to its nature (see Table C). This reagent, is often used for detecting— magnesia, which gives a pale red colour; alumina, which gives a blue without lustre.


Blowpipe Analysis Confirmatory tests when the mineral has been treated alone on charcoal or with carbonate of soda:
(i.) When metallic beads or spangles are left:
Silver.—If dissolved in nitric acid, an addition of hydrochloric acid or a solution of common salt will precipitate white chloride of silver.
Gold.—If dissolved in 4 parts hydrochloric acid and 1 part nitric acid, a precipitate of purple of Cassius will be obtained when protochloride of tin is added.
Copper.—If treated with borax on platinum wire it will give reactions, as in Table A.
(ii.) When a grey or blackish residue is left:
Heat the residue with borax on platinum wire and note the colour of the bead; compare results with Table A, for COBALT, COPPER, IRON, NICKEL.
(iii.) When the mineral yields an incrustation on the charcoal:
Antimony.—If the scraped-off incrustation be treated with hydrochloric acid and zinc on a piece of platinum foil, a black film of antimony is formed.
Lead.—If dissolved in nitric acid, the excess of acid evaporated and a little sulphuric acid be added, a white powder will be formed.
Tin.—If dissolved in hydrochloric acid, a grey precipitate is formed when metallic zinc is placed in the solution.
Zinc.—If the incrustation be heated with the nitrate of cobalt solution, it becomes green.
To detect certain common substances associated with metals:
Alumina.—This is known by its adhering readily to the tongue when licked. Tested before the blowpipe with nitrate of cobalt, it becomes blue.
Lime.—This gives a very bright light when heated before the blowpipe flame. It is infusible even with carbonate of soda, and so differs from silica and flinty substances.
Carbonate of Lime effervesces when a little hydrochloric or citric acid is dropped on it.
Magnesia.—When heated with nitrate of cobalt solution, becomes flesh red.
Soda.—When strongly heated, gives a reddish yellow colour to the outer flame.
Potash gives a violet colour to it.
Sulphur is known by its characteristic odour when the substance is roasted. If a portion of the heated mineral be placed on a moistened piece of silver, a black stain shows the presence of sulphur.
Arsenic is known by its characteristic garlic odour when heated.
All carbonates effervesce in acids. (N.B. A limestone rock, which is made up of carbonate of lime, can thus be easily distinguished from a sandstone). Certain silicates, when acted on by acid and heated, gelatinize.
Blowpipe Assay
The Blowpipe Assaying method, though less exact than the furnace methods, is of importance, because in prospecting expeditions it is possible by its means not only to detect the gold and silver in any ore, but also to determine its amount quantitatively with fair accuracy. On such expeditions it is impossible to carry the cumbrous apparatus required to make an ordinary assay. The amount of powdered ore taken is usually 100 milligrammes, and this is mixed with borax and about 1 gramme of granulated lead. The whole is wrapped in paper and heated on charcoal in the reducing flame of a blow¬pipe until the fusion is complete, and then for a short time with the oxidising flame. The lead is then separated from the slag and heated on a bone-ash cupel until it is all converted into litharge. The diameter of the button of silver and gold thus obtained is carefully measured on an ivory scale, which at once gives the percentage amount in the ore. The gold is usually separated from the silver by parting in nitric acid, but Richards states that the silver can be distilled off by the blowpipe, leaving a bead of pure gold, which can be measured.
How to make a blowpipe for temporary use in mineralogical analysis
Procure a long pipe of glass tube (1/3 inch thick). Hold it horizontally over the flame of a spirit lamp. As the middle part becomes softened pull both ends of the tube horizontally, until the middle part of the tube is about the thickness of an ordinary blowpipe jet. File a notch on the thin part, and snap the tube. Now take one portion and again heat it (a little distance from the nozzle), and bend it so that the nozzle may be inclined at an angle to the longer branch.
[/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]
