Some of us who have been acquainted with the present methods of production of metals, and who have had an opportunity to witness the impact of some of the newer chemical techniques on metallurgy, have become convinced that it is possible to consider seriously the treatment of sulfide minerals by strictly chemical methods as distinct from pyrometallurgical methods. In other words, a chemical smelter. The authors of this paper believe that profit making flowsheets can be developed for chemical recovery of base metals from sulfide ores with 95% recovery of contained gold, silver, copper, lead and zinc. A major advantage of such a possibility is the fact that no prior separation would be required and that very fine grained and complex ores could be treated economically.
Such a conviction does not really amount to much more than loose talk until someone sits down and begins to put dollar signs on the chemistry.
It is our purpose to show the results of estimates which we have made on the cost of treating the most difficult sulfide ore we could dream up as feed for our chemical smelter. This ore is assumed to have the following analysis:
Gold………………………………………………0.2 oz. per ton
Silver……………………………………………8.00 oz. per ton
Copper……………………………………………0.50%
Lead……………………………………………….6.00%
Zinc……………………………………………….11.00%
Arsenic…………………………………………..2.50%
Iron……………………………………………….20.00%
Sulfur……………………………………………..30.5 %
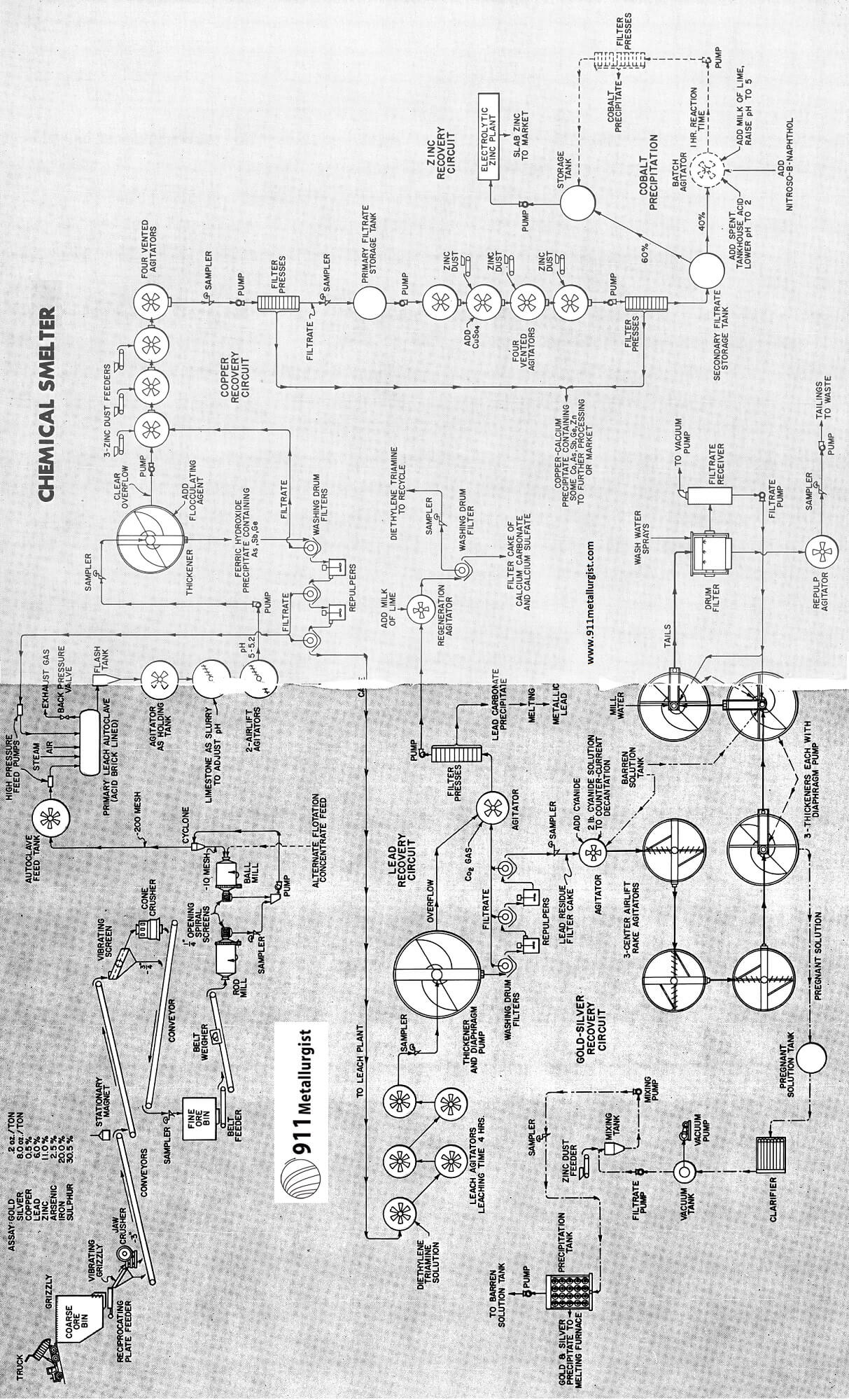
General Chemical Smelter Flowsheet
Briefly, the flowsheet upon which we have based our calculations assumes that the entire ore would be oxidized in a pressure leach autoclave, using air for oxidation. This would result in converting all of the copper and zinc to the soluble copper and zinc sulfates, and converting the lead to insoluble lead sulfate. It is anticipated that neither the gold nor silver would be solubilized by this treatment. This oxidation would also produce sulfuric acid from the pyrite.
After the autoclave leach the excess acidity would be neutralized with limestone and the slurry filtered, thereby producing a solution containing the copper and zinc as sulfates. The copper would be recovered from this solution by precipitation with zinc, and the zinc would be recovered by electrolysis.
The lead sulfate in the solid tails would be dissolved with a solution of diethylene triamine. The pregnant solution of triamine would be separated from the solids by filtration and the lead recovered as very high purity lead carbonate by precipitation with carbon dioxide. Because of its very high purity it could be reduced directly to metallic lead in a melting furnace to produce 99.99% lead metal.
After removal of the lead from the tailings the residue would be cyanided for recovery of gold and silver.
There is no part of our proposed flowsheet which has not been demonstrated, either in laboratory tests or in full scale production. There is adequate information from commercial operations to estimate the cost of recovery of copper and zinc from acidic sulfate solutions, the cost for recovery of gold and silver is well known, and autoclave leaching under pressure is a relatively common large scale operation nowadays. Therefore the cost for most of the steps can be estimated with fair accuracy. As a basis for our cost calculation we have assumed that our chemical smelter will treat 300 tons per day of the ore of the composition given.
Autoclave Leaching
The treatment starts out by grinding the ore to — 200 mesh and oxidizing it in pressure leach,autoclaves, using air for oxidation. The actual operation of a pressure leach circuit handling —200 mesh sulfide in strong sulfuric acid solution at high temperatures and pressures has been successfully demonstrated at several commercial installations.
The chemical reactions in the pressure leach are exothermic and no external heat would be needed. In fact, cooling coils would be needed if it were not for the additional solutions pumped into the autoclaves to make up for the water in the blow-off gases.
The following equations indicate the overall results of this treatment.
CuS + 2 O2 → CUSO4
PbS +2 02 → PbSO4
ZnS + 2 O2 → ZnS04
Pyrite+oxygen+water+n H2SO4+X Fe2(S04)3 .y Fe2O3 *z H2O
The leached pulp discharged from the autoclaves would have in solution all of the acid made from the ore as well as the acid in solution recycled from the zinc plant, plus 99% of the zinc and the copper originally in the 300 tons of ore.
Filtration would be done over three washing filters in series. The filtrate would go to the zinc plant for purification, recovery of cement copper and electrolytic recovery of the zinc. The washed filter cake would go to the lead circuit for recovery of lead.
Direct mill costs up to this point would total $7.00 per ton of original ore treated. These costs would include crushing and grinding as well as the pressure leaching. Of this cost, $4.00 per ton of ore would be spent merely to compress air for the autoclaves. This circuit would require 26 men and use 115,200 KWH per day or 384 KWH per ton of feed ore.
Recovery of Copper and Zinc
The filtrate from washing the leached autoclave pulp is the raw pregnant solution delivered to the zinc plant. Including the recycle, this solution would have the following probable analysis:

In the first step of the purification this solution is raised to a pH of about 5 with lime. The iron would precipitate as ferric hydroxide and co-precipitate the bulk of the arsenic, antimony and germanium in solution. The clarified solution would then be treated with zinc dust to precipitate the copper as well as any cadmium that might be present.
The copper precipitate would be sold as cement copper because there isn’t enough copper in this ore to justify its recovery as either electrolytic copper or as hydrogen-reduced copper powder.
Zinc is recovered by electrolytic process.
Recovery of Lead
The washed filter cake from the autoclaves would be pulped and agitated for four hours at ambient temperature with an aqueous solution containing 50 gpl of diethylene triamine.
After filtration pure white basic lead carbonate would then be precipitated from the filtrate bl blowing CO2 gas into the solution.
Recovery of Gold and Silver
Washed lead residue still contains all gold and silver originally in the ore. We leach this pulp in a conventional cyanide agitation circuit. After being agitated in a 2-pound cyanide solution, the pulp would be washed countercurrently over washing filters. The washed residue would be pumped to the tailing pond. The gold and silver in the filtrate solution would be precipitated with zinc dust and the barren solution recycled in the usual manner. The gold-silver precipitate, containing 95% of the gold and silver, would be fluxed and melted and cast into dore’ bullion bars. We assume that the ore will consume 5 pounds of cyanide per ton and that 16 employees would be needed to operate the circuit. The total direct operating costs would amount to $3.00 per ton of original ore.
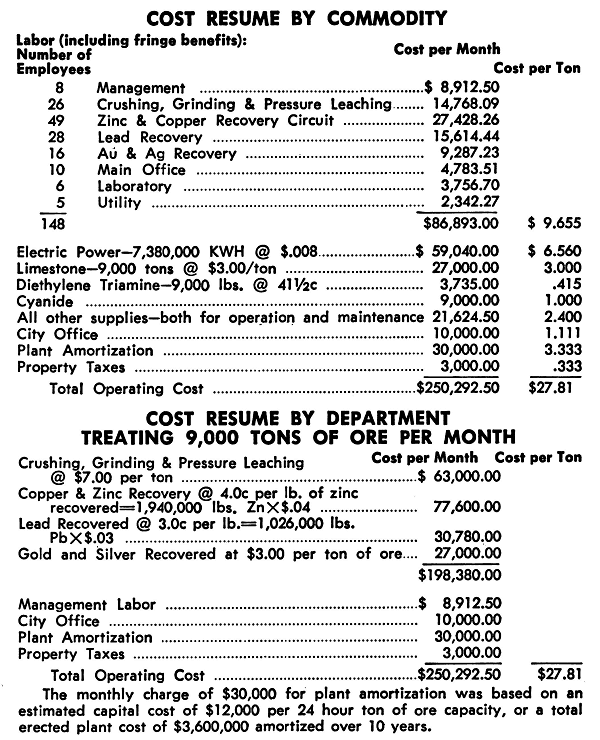
Total Operating Cost
We estimate that the total cost of treating 300 tons of this ore per day and recovering 95% of each metal would amount to $27.81 per ton of ore treated. The estimates are summarized on both the commodity basis and by departments in the following tabulation:
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.