As coarse gold is not dissolved by cyanide, it must be removed from an ore by one of the methods already described. Weak solutions of sodium cyanide (or potassium cyanide, seldom used) will dissolve the fine, untarnished gold and silver in ores and tailings, provided the latter have been ground fine enough and do not contain any material, such as acid compounds or minerals, that would affect the cyanide. The gold and silver are recovered or precipitated from the solutions by means of zinc shavings or zinc dust. Melting of the dried precipitate with fluxes yields marketable bullion. An entire ore may be slimed and cyanided, or it may be divided into sand and slime and then cyanided. Tailings may be sand or slime or a mixture of such. Barren solutions, when strengthened with more cyanide salt, can be used again. The strength of solutions must be known, but this is easily determined, as described later.
Cyanide
Two types of cyanide are used in treating gold and silver ores and tailings, also in the flotation of ores: sodium cyanide, which contains 98 per cent NaCN, and calcium cyanide (Aero brand), which contains up to 50 per cent NaCN equivalent. The wholesale price for these cyanides has been steady around 16 to 18 cents or 8 to 9 pence a pound for the sodium salt and 13 to 15 cents or 6½ to 7½ pence for the calcium salt per pound of actual contained sodium cyanide equivalent. As a rule, one cyanide is as effective as the other, but more of the calcium salt must be used because of its lower strength.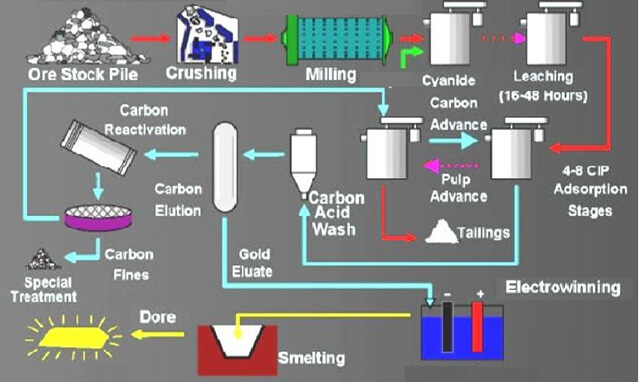
Cyanides should be purchased from chemical supply firms. The sodium salt is made in the form of small cubes or in blocks or cakes packed in drums. The calcium salt is made in the form of black flakes and is packed in drums holding 100 and 200 pounds.
Both forms of cyanide adsorb moisture on exposure and become slippery. Both are easily soluble in water, the calcium salt leaving a little undissolved material in the dissolving tank.
(Cyanides in any form—as salts, as strong and weak solutions, and as vapors in enclosed places—are deadly; precautions are necessary at all times when handling these forms of cyanide.)
Cyanide may be added to water to form solutions or to strengthen weak solutions by dissolving part of a drum or several drums of it in a special stock or stronger-solution tank or by placing the salt at some point in a launder or in a basket at the discharge of a pipe where pulp or solutions are flowing. When a stock tank is used, the solution’s strength must be known before any is mixed with weak solutions; and when the salts are dissolved by flowing material, the amount used must be calculated to give the propel strength.
Water
An important factor in cyanidation is the quality of the water used. At most places this does not occasion any trouble, but in others it may be brackish, salty, acid, or high in magnesia. The acidity or alkalinity of water is simply determined by means of litmus papers, as explained in another part of this book. Milling, cyaniding, and floating can be and is done satisfactorily in brackish or salty water. If acid, the water must be neutralized with lime, even before milling and especially before amalgamation. In parts of Australia and Rhodesia the water is high in magnesia. This increases the consumption of cyanide, but the addition of lime precipitates the magnesia and leaves the water slightly alkaline. For example, 8, 12, 15, 22, 28, and 40 pounds of lime have been added per ton of water to precipitate the magnesia; and at one mine, where 12 pounds was required, the cyanide used was cut from 2 pounds to 1/3 pound per ton of ore treated. Therefore, the quality of the water must be watched.
Solutions
Clean gold ores may be treated with first solutions having a strength of 0.05 per cent or less NaCN and with a consumption of 1 pound or less per ton treated. If silver occurs with the gold, the strength and consumption will be somewhat higher. Straight silver ores require a solution strength of 0.3 per cent or more NaCN and consume twice as much cyanide as do gold ores. Concentrates may need solutions as strong as 0.1 to 0.8 per cent NaCN, depending on the silver, and the consumption may reach several pounds per ton.
Cyanide solutions may become more or less fouled by the decomposition of minerals during treatment, this forming soluble sulphides. If this condition arises, they may be rendered harmless by adding lead salts in the form of lead acetate, lead nitrate, or lead oxide (litharge). These salts may be added at any point in the plant, but the proper time must be determined by trial. The quantity consumed is small.
Cyanide solutions must be kept alkaline, and lime is added at various points in a plant, including to the ore as fed to the mill.
The first solutions used in a plant are made up of fresh water (salt water will do if it is the only water available) and sodium or calcium cyanide, but all new strong solutions are made up of weak barren solutions and either the cyanide salt (98 per cent) or a strong solution from a stock tank containing a 10 to 20 per cent cyanide. A calculation involving the strength of the salt or stock solution, of the barren solution, and of the desired solution, also the quantity of the solution needed in tons, gives the amount of salt or stock solution to be added.
It will require 2 pounds of sodium cyanide for each ton of water to make a solution testing 0.1 per cent cyanide or half of that amount for an 0.05 per cent solution. If weak solutions are used in the make-up, the salt required will be less than these amounts.
How to Test Solutions of Cyanide
Solutions of cyanide are tested by the silver-nitrate method, as follows:
- Dissolve 13.08 grams of silver nitrate in 1000 milliliters or 1 liter of distilled water. (If only occasional tests are to be made, take half or a quarter of these quantities or buy the solution ready made.) Keep the solution in a dark bottle because it decomposes in the light.
- Dissolve 10 grams of potassium iodide in 100 cubic centimeters of distilled water.
- Two 50-cubic centimeter burettes on one stand or two close together are required.
- Fill the burettes—one with the cyanide solution to be tested and the other with the silver-nitrate solution.
- Measure out 10 cubic centimeters of cyanide solution into a small beaker, add to it two or three drops of potassium iodide, and then slowly drop in silver nitrate. A whitish turbidity shows but re-dissolves as long as any free cyanide is present. The beaker should be shaken. The end-point is a permanent faint white to bluish turbidity.
- Note the amount of silver nitrate used and calculate: 1 milliliter of silver nitrate = 0.1 per cent cyanide or 2 pounds of cyanide per ton of solution.
