In the Cyanide Destruction by Hypochlorite reaction, the pH has a strong inverse effect on the ORP. Thus, wastewater treatment facilities must closely control the pH to achieve consistent ORP control, especially if they use hypochlorite as the oxidizing agent. Adding hypochlorite raises the pH, which, if unchecked, lowers the ORP. calling for additional hypochlorite. Controlling the pH at a setting above the pH level where hypochlorite has an influence and separating the ORP…
Alkaline Chlorine-Hypochlorite Oxidation: Chlorine was used for cyanide destruction in the early days of cyanidation in the late 1800s, because chlorine and its derivatives were readily available in the industry at that time. The method has been applied ever since in a variety of forms.
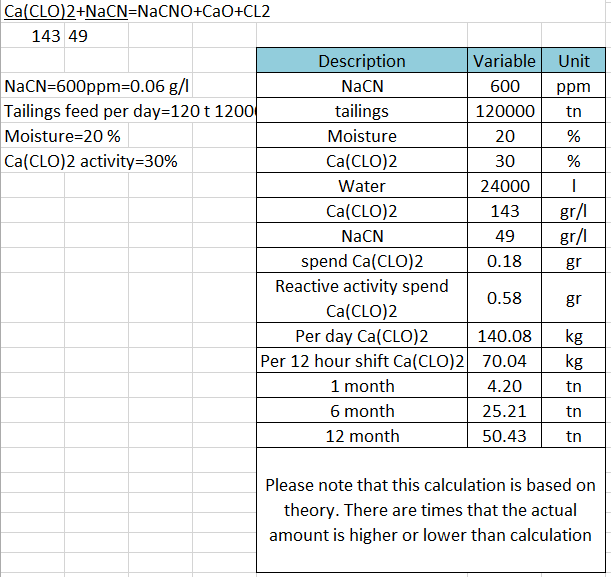
The active reagent for chlorine oxidation of free and complexed cyanide is the hypochlorite ion, produced when chlorine dissolves in water, as described. Alternatively, hypochlorite ions can be produced by dissolving suitable salts, such as sodium or calcium hypochlorite, in water.
Free cyanide reacts rapidly with hypochlorite (OCl) in aqueous solution to form cyanogen chloride, otherwise known as tear gas.
Cyanide also reacts rapidly with free chlorine.
However, at high pH, cyanogen chloride is readily hydrolyzed to cyanate and chloride ions.
In practice, effective oxidation of cyanide can be achieved in 10 to 15 min. The rate of the initial oxidation reaction shown in Equation (11.25) is reduced significantly as the pH is increased >11, and the oxidation process is usually carried out at pH 10 to 11, which is high enough to avoid significant cyanogen chloride formation. The hydrolysis reaction consumes hydroxide, and supplemental alkali must be added if the feed to chlorination is insufficiently buffered. The use of sodium or calcium hypochlorite may obviate the need for alkali addition.
If the hypochlorite ion concentration is sufficiently high, nitrogen and carbon dioxide may be produced.