A few representative cycles from the first 20 oxidation/reduction cycles for a pyrite electrode in 0.1 M sodium borate are presented in Figure 3. The first scan was made in the cathodic direction starting from the rest potential of the mineral which typically had a value of 0.18 V. The large reduction peak observed in the first cycle shows the presence of oxidation products on the pyrite surface. These oxidation products are formed during the polishing of the pyrite electrode prior to its insertion into the solution. The anodic current in the first cycle is considerably smaller than the cathodic current which implies that the species reduced during the first cathodic cycle are not formed in the duration of the anodic scan. This is confirmed by a considerable decrease in the current in the second cathodic cycle. The reduction peak potential also shifted to less cathodic potentials. Such a shift in potential is associated with a change in the type of species being reduced.
Several investigators have studied the oxidation of pyrite and proposed that several solid products Fe(OH)3, FeOOH, Fe3O4, Fe2O3, etc., may form (Hiskey ana Schlitt, 1981; Nordstrom, 1982), Reduction of such oxides gives rise to the reduction peak during the first scan. During subsequent scans iron oxidizes only to Fe(OH)3 which is reduced to Fe(OH)2 in the reduction cycle.
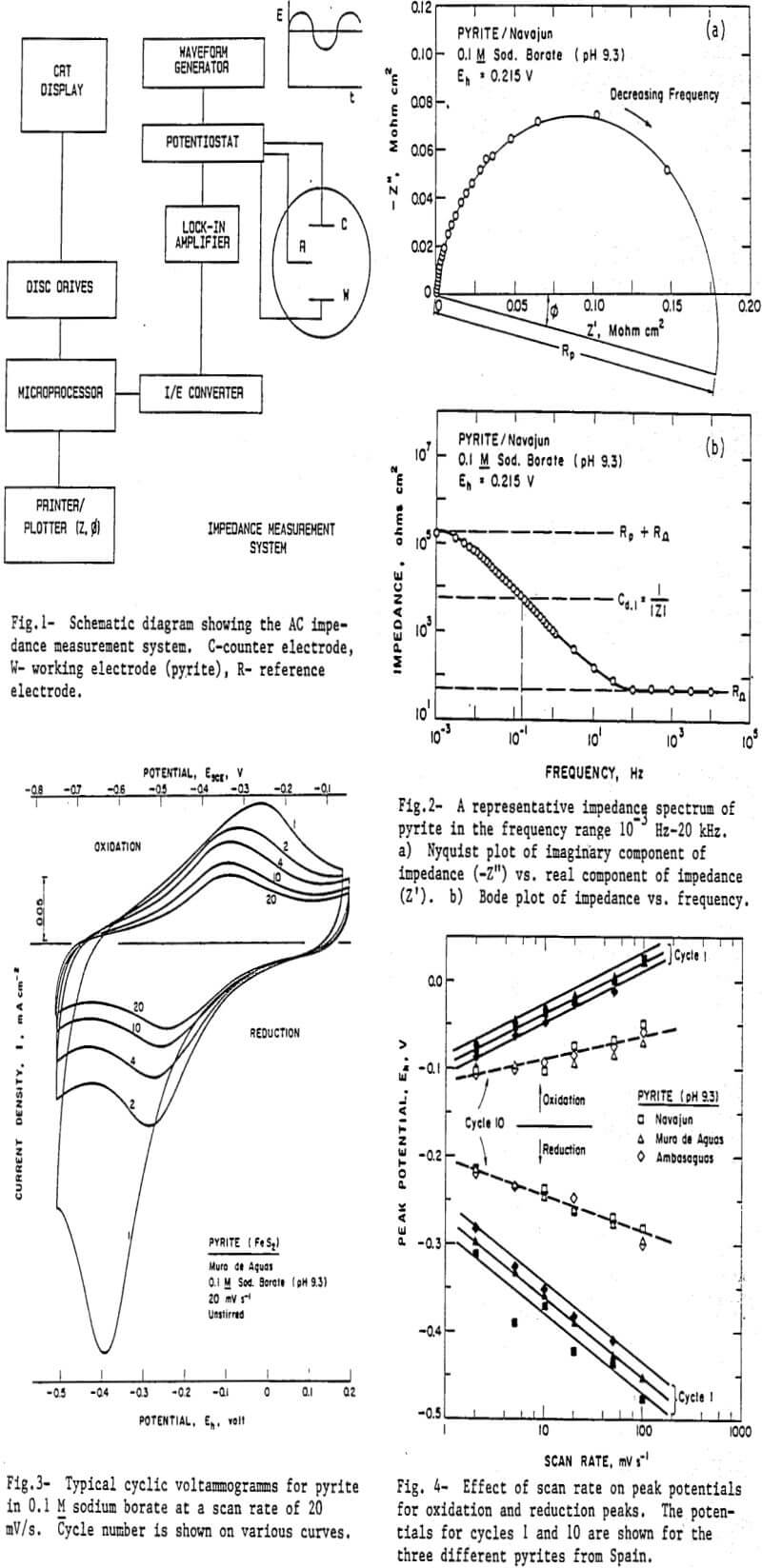
To further study the nature of the oxidation/reduction reactions, the effect of scan rate on the oxidation and reduction peaks was determined and the results are presented in Figures 4 and 5. The data for cycles 1 and 10 are given. Cycle 1 is taken as representative of the initial state of the electrode whereas cycle 10 is taken as a representative of the steady state condition of the electrode under the conditions of experimentation. The potentials corresponding to peak currents are plotted as a function of scan rate in Figure 3. The following observations may be made regarding the oxidation/reduction reactions on pyrite:
- the oxidation and reduction peaks are separated by a large potential difference,
- the peaks potentials for both the reduction and oxidation peaks are a function of scan rate, and
- the peak current is a non-linear increasing function of the square root of scan rate.
These observations suggest that the reactions are irreversible.
The slope of the potential vs. log (scan rate) plot is greater for the first cycle compared to the subsequent cycles (represented by cycle 10) which are interpretted to mean that the degree of irreversibility is more for the substance that initially forms on the pyrite surface during the polishing procedure. After the first reduction scan, the initial product(s) of oxidation transform into Fe(OH)2 which oxidizes to Fe(OH)3 during the anodic scan. In subsequent cycles FeOH)3 is reduced to Fe(OH)2. Oxidation of pyrite does not occur in the potential range used in the scanning experiments. The surface remains covered, with a substance produced during the polishing procedure, however. This substance transforms into Fe(OH)2 or Fe(OH)3 during repeated cycling of the electrode.
The reduction peak potentials for cycle 1 are dependent on the source of pyrite and increased in the order:
Ambasaguas < Muro de Aguas < Navajun
The differences between the reduction peak potentials for pyrites from various sources observed in cycle 1 diminish in subsequent cycles. The quantity of the surface species also decreases as seen by decrease in the reduction current with increase in number of cycles. The decrease in current was maximum from cycle 1 to cycle 2 and more gradual thereafter. In Figure 5, the currents for cycle 1 and 10 are plotted for the sample from Navajun only. A similar decrease in current was observed for other pyrite samples. In previous studies of electrochemistry of pyrite electrode Hamilton and Woods (1984) postulated that the reaction on pyrite may be written as:
Fe(OH)2 + OH- = Fe(OH)3 + e-…………………………………………………..(6)
The decrease in current on repeated cycling may be attributed to a decrease in the effective surface area of the electrode as proposed by Mitchell and Woods (1978). In addition, the decrease in current from cycles 2 to 20 was not affected by solution stirring indicating that the reaction involves surface species. The results presented in Figures 4 and 5 show that the potentials and currents are a function of the pyrite source. The electrode preparation and solution conditions were kept the same as far as possible. These results correlate very well with the impedance measurements presented in the next section and are considered to be inherent to the mineral.

