The de-silverizing of base bullion is carried out in accordance with the principles of the well-known Parkes process.
The base bullion produced by the blast furnace department carries (apart from silver values) impurities, the chief of which are copper, antimony, and arsenic.
In order to more clearly indicate the grade of the base bullion, the following analysis, representative of a half-year’s production of base bullion, is submitted:
Ag………………………………………………..68.6 oz.
Au………………………………………………0.096 oz.
Cu………………………………………………….0.86%
Sb………………………………………………….0.67%
As………………………………………………….0.32%
Before proceeding with the actual operation of de-silverizing, it is necessary that the impurities, copper, antimony, and arsenic, should be eliminated. This is accomplished in the following steps :
Removal of Copper from Bullion
Copper is largely present in the base bullion as dissolved sulphide, and this is removed by liquation in a reverberatory type furnace, of which the following are the essential details:
Hearth area…………………………………220 sq. ft.
Grate area……………………………………..31 sq. ft.
Ratio hearth area to grate area……………….7:1.
Capacity…………………………………………57 tons.
Working temperature………………..750° to 850° C.
Coal consumption per 24 hours…………2 tons.
The hearth of the furnace is encased in a steel tank 22 ft. 4 in. long by 13 ft. 4 in. wide by 2 ft. 10 in. deep ; the bottom of the tank is covered by a depth of composition conforming to the invert:
Cement………………………………39 cwt.
Brick dust………………………..8.4 tons.
Fireclay……………………………0.6 tons.
Sand………………………………..0.6 tons.
When the bullion is melted in this drossing furnace a quantity of sulphide dross, amounting to approximately 8% of the weight of the charge, floats to the surface. To free this dross from entangled lead it is necessary to fire the charge to about 800° C. At this temperature it is probable that a roast reaction is set up, liberating some metallic copper, which becomes alloyed with the bullion. The method of eliminating this metallic copper will be discussed at a later stage.
The dross now remaining on the surface of the charge is skimmed off and eventually treated in a small blast furnace, when the greater part of the entangled lead is recovered, while a Cu-Pb matte is produced. By this means the copper is eliminated from the circuit.
BULLION
A normal time-table of the copper drossing furnace is as follows:
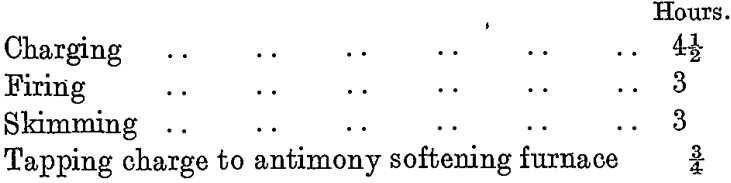
A typical analysis of the copper dross obtained from this furnace is as under:

The bullion, after copper dressing, is of the following typical analysis :—
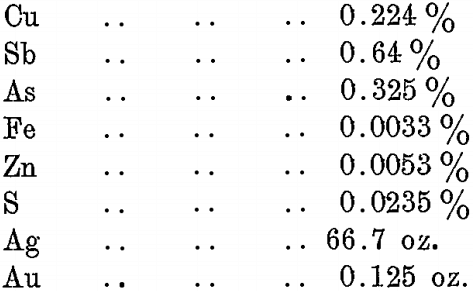
Removal of Antimony and Arsenic
The furnace used for the removal of antimony and arsenic is a replica of the copper-drossing furnace, except that a water girder is placed in at the lead level, with a notch in the end girder, by which the dross is run off the charge. The working temperature of the antimony-softening furnace is from 1000° to 1100° C. This furnace is filled by gravitation from the copper-drossing furnace.
The normal cycle of operations on this furnace is :—
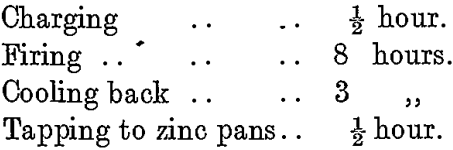
The products from this furnace are:
- Antimonial dross—about 2 tons from each charge.
- Softened bullion.
And the following are typical analyses of the respective products:
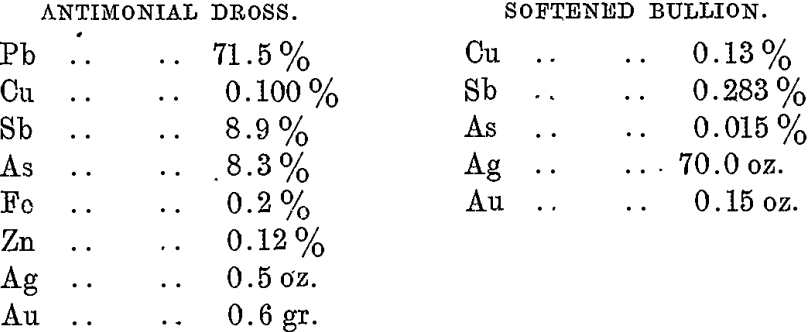
In this furnace the antimonial and arsenical skimmings flow continuously through the notch in the end water girder, and are eventually treated in the antimony dross furnace. This furnace produces:
(a) Hard lead.
(b) Antimonial slag.
The former product is re-treated in the antimony softening furnace, being slowly worked off with the current softened bullion, while the latter is treated in a small blast furnace producing antimonial lead and waste slag.
As indicated above, the normal cycle of softening a charge is 12 hours.
In the softening process the arsenic is removed at a much faster rate than is the antimony. This is illustrated by the following experiment, in which the antimony and arsenic content of the bullion was determined before firing and at intervals of 6 hours until the charge was clean.:—
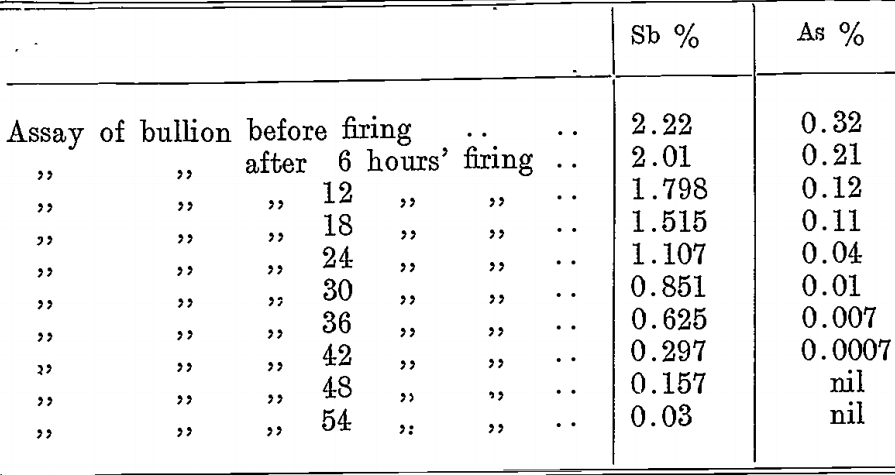
Provided arsenic is not present to the extent of several per cent.; it is readily eliminated in the softening furnace, being a readily- oxidized metal.
Consider now the effect of antimony and arsenic in the softened bullion in connection with the de-silverizing operation.
Antimony may be present to the extent of 0.5 % without seriously interfering, but 1.0% has been found to have the effect of reducing the quantity of silver removed by a given quantity of zinc.
Arsenic, if present to the extent of a few tenths of a per cent., obscures the line of demarcation between the zinc crusts and the underlying bullion, owing to the arsenic entering the crust and making a mushy crust.
It will be shown later that the cycle of the zincing kettle is 24 hours, so on this account 2 zincing kettles are installed to each antimony softening furnace.
ZINCING KETTLES
The zincing kettles are of hemispherical section, being 10 ft. 8 in. diam. internally by 3 ft. 7 in. deep at the centre, and are made of cast- iron, 1¾ in. thick throughout. The kettle is cast with a flange 6 in. wide by 1¾ in. thick, which serves as the support of the pan in the brickwork setting. The setting of the kettle is of freestone and brickwork surmounted by an annular ring of iron on the brick wall which supports the kettle by the flange.
These kettles have a capacity of 57 tons of bullion. They are direct-fired, having a grate area of 17½ sq. ft., and consume about 25 cwt. of coal per 24 hours.
The life of the zincing kettles depends to some extent on the method of firing, but to a very large degree on the quality of the cast-iron from which the kettles are manufactured.
It will be shown later that in the actual zincing operation the 57-ton charge of bullion has to be cooled back to solidification point four times and raised to approximately 450° C. three times in 24 hours. Consequently, rapid firing and cooling are essential if the cycle is to be run to time.
In the writer’s experience, the average life of zincing kettles was increased from 8 to 13 months by care being exercised in the grade of iron from which the kettles were cast.
CYCLE OF OPERATION IN DE-SILVERIZING
As mentioned previously, there are two zincing kettles to each antimony softening furnace, and for convenience in outlining the cycle these kettles will be called “ A” and “ B.”
As soon as kettle “ A ” is charged by gravitation from the softening furnace a quantity of dross, which forms on the surface of the lead, is skimmed off. This dross is returned to the copper-drossing furnace.
The charge is now cooled back to the solidification point, and the Pb-Cu alloy, or cold dross, referred to under “ Removal of Copper,” is removed and likewise returned to the copper-drossing furnace.
A typical analysis of this dross is as follows :—
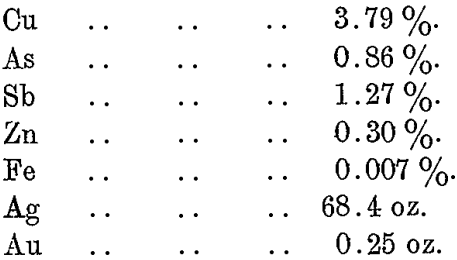
This “ cold dross ” is added to the copper-drossing furnace when the latter is nearly charged and covered with a sulphide dross. The metallic copper then combines with the sulphur and passes off with the copper dross from the furnace.
The following analysis is typical of the bullion after the removal of the “hot” and “cold” dross:—

The average time taken from the commencement of charging the kettles to the removal of the cold dross is 4½ hours.
The kettle is now strongly fired, and 325 lb. of zinc are added to the charge, and when the zinc is melted the whole charge is mechanically stirred thoroughly for 30 minutes. From the finish of cold drossing to the completion of the stirring-in process 2 hours are allowed.
This first addition of zinc (325 lb.) is known as “ gold zincing.”
Owing to the small amount of gold present in the bullion it is necessary, from-the point of view of eventually recovering this gold, to obtain in the first or gold-zincing the maximum of gold and the minimum of silver.
This first or gold zincing takes up as well any copper which remained in the bullion after hot and cold drossing.
After the charge has been thoroughly stirred, the fire is drawn and the charge allowed to cool back. In this operation a crust is formed containing gold, copper, silver, and zinc. This is skimmed off with perforated skimmers, and the operation is so timed that the charge is just beginning to solidify when the final crust has been removed.
The time taken from the completion of the stirring in of the zinc to the removal of the gold crust is 4 hours.
The gold crust above referred to, together with 325 lb. of zinc, is now returned to kettle “ B ” as a gold-zincing.
Kettle “ B ” has been charged from the antimony softening furnace 12 hours after kettle “ A.”
The operation on kettle “ B ” is identical with that described in connection with kettle “ A.”
Again, the gold crust from kettle “ B,” together with 325 lb. zinc, is returned as the first or gold zincing to the next charge delivered to, kettle “A.”
The gold crust from this new charge in kettle “A” is now, together with 325 lb. zinc, returned as the first or gold zincing to the new charge in kettle “ B.”
The object of this four-cycle operation of the gold-zincing is to concentrate the gold from four charges of bullion into one crust.
After the fourth cycle the gold crust is pressed in the Howard press in order to remove the entangled lead and ensure a minimum tonnage of gold crust, which has to be further treated.
After the gold-zincing has been completed the temperature of the charge is raised to a degree sufficient to melt zinc and the first silver-zincing given.
While the kettle is thus firing, the crusts from a previous second silver-zincing, amounting to approximately 5 tons, are added to the charge. As soon as the temperature necessary to melt zinc is reached, the charge is mechanically stirred for 30 minutes. The crust from a second silver-zincing, referred to above, will be explained later.
After the stirring is completed the fire is drawn, and the charge allowed to cool back.
The crust which forms is removed by the Howard press, and the operation is so timed that when the final crust is removed the bullion just begins to solidify.
During the pressing of the crust the sides of the kettle are carefully scraped down in order to remove any alloy or crust adhering to the kettle.
When the available crust has been removed, that portion of the charge which has solidified around the edge of the kettle is pushed off into the molten bullion. Upon melting it will give up any alloy which it may have contained. This alloy is skimmed off by hand into moulds and added to the next kettle before the next silver-zincing.
The pressed crust referred to above carries approximately 1850 oz. per ton, and is now retorted and cupelled, producing silver of 998.5 fineness.
After the removal of this silver crust the charge is again brought to a temperature at which zinc will readily melt, and a second silver- zincing of 1100 lb. zinc given.
It is essential that the silver in the charge after the second zincing must not exceed 4 dwt. Ag per ton. Should the charge contain more than 4 dwt. Ag per ton an extra zincing must be given. This takes 6 hours, and, consequently, should this extra zincing be required, it is obvious that the rest of the unit is at a standstill for that period. Under these conditions it is necessary to add zinc in such quantities as to minimize the chance of a miss. This practice also provides so much available zinc in the final crust that, when used as a first silver-zincing on the next charge, the bullion will be reduced to 6-10 oz. silver per ton.
After the 1100 lb. of zinc has been stirred in, the charge is taken right back to the solidification point, and extra care is taken to ensure the last trace of alloy being removed, the kettle being scraped down and skimmed at frequent intervals. The charge is now heated up, and during the heating process the kettle is again scraped down to remove the last trace of crust.
A silver determination is now made on the charge, and if the result is within the 4 dwt. limit of silver the charge is dropped to the refining furnace. At this stage the lead contains 0.6 % zinc, this being the Pb-Zn eutectic.
In the refining furnace zinc and traces of other impurities are oxidized, forming a dross, which is skimmed from the surface of the bath of lead. A typical sample of this dross shows:—
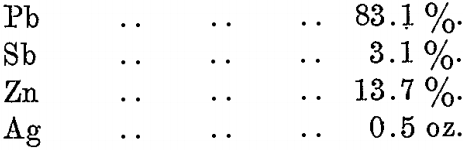
The zinc remaining in the lead after de-silverizing is wholly lost, and amounts to 13.4 lb. zinc per ton of lead. This loss is in no way dependent on the amount of silver in the bullion being treated, being a constant loss whether the silver be 1 oz. per ton or 100 oz. per ton.
A further quantity of zinc is lost in the retorting of the silver crusts, the average recovery being around 75 % of the total zinc held in silver crusts.
In the case of the Port Pirie practice a further zinc loss is sustained in the scorifying of gold crusts, since the gold in the bullion is so small as to require selective gold-zincing, and prevents a first gold-silver crust being obtained in which the ratio of Au to Ag would be such as to permit the final dross from these crusts being economically parted.
