Scope of Descriptive Mineralogy. — It is the province of Descriptive Mineralogy to describe each mineral species, as regards: (1) form and structure; (2) physical characters; (3) chemical composition including blowpipe and chemical tests; (4) occurrence in nature with reference to geographical distribution and association with other species; also in connection with the above to show how it may be distinguished from other species. Further, it should classify mineral species into more or less comprehensive groups according to those characters regarded as most essential. Other points which may or may not be included are the investigation of the methods of origin of minerals; the changes that they undergo in nature and the results of such alteration; also the methods by which the same compounds may be made in the laboratory; finally, the uses of minerals as ores, for ornament and in the arts.
Scheme of Classification. — The method of classification adopted in this work, and the one which can alone claim to be thoroughly scientific, is that which places similar chemical compounds together in a common class and which further arranges the mineral species into groups according to the more minute relations existing between them in chemical composition, crystalline form and other physical properties.
Upon this basis there are recognized eight distinct chemical classes, beginning with the Native Elements; these are enumerated on the following page. Under each of these, sections of different grades are made, also based on chemical relationships. Finally, the mineral species themselves are arranged, as far as possible, in isomorphous groups, including those which have, at once, analogous chemical composition and similar crystallization (see Art. 471). It is unnecessary to take the space here to develop the entire scheme of classification in detail, since a survey of the successive sub-classes under any one of the divisions will make the principles followed entirely clear. A few remarks, only, are added for sake of illustration.
Under the Oxides, for example, the classification is as follows: First, the Oxides of silicon (quartz, tridymite, opal). Second, the Oxides of the semi-metals, tellurium, arsenic, antimony, bismuth, also molybdenum, tungsten. Third, the Oxides of the metals, as copper, zinc, iron, manganese, tin, etc. The third section is then subdivided into the anhydrous and hydrous species. Further, the former fall into the four divisions: Protoxides, R2O and RO; Sesquioxides, R2O3; Intermediate oxides, RO,R2O3; Dioxides, RO2. Under each of these heads come finally the individual species, arranged so far as possible in isomorphous groups. Thus we have the Hematite group, the Rutile group, etc.
In regard to the various classes of salts it may be stated that, in general, they are separated into anhydrous, acid, basic and hydrous sections; the special subdivisions called for, however, vary in the different cases.
For an explanation of the abbreviations used in the description of species, see p. 5.
SCHEME OF CLASSIFICATION
I. NATIVE ELEMENTS.
II. SULPHIDES, SELENIDES, TELLURIDES, ARSENIDES, ANTIMONIDES.
III. Sulpho-salts. — SULPHARSENITES, SULPHANTIMONITES, SULPHOBISMUTHITES.
IV. Haloids. — CHLORIDES, BROMIDES, IODIDES; FLUORIDES.
V. OXIDES.
VI. Oxygen Salts.
- CARBONATES.
- SILICATES, TITANATES.
- NIOBATES, TANTALATES.
- PHOSPHATES, ARSENATES, VANADATES; ANTIMONATES. NITRATES.
- BORATES. URANATES.
- SULPHATES, CHROMATES, TELLURATES.
- TUNGSTATES, MOLYBDATES.
VII. Salts of Organic Acids: Oxalates, Mellates, etc.
VIII. HYDROCARBON COMPOUNDS.
I. NATIVE ELEMENTS
The NATIVE ELEMENTS are divided into the two distinct sections of the Metals and the Non-metals, and these are connected by the transition class of the Semi-metals. The distinction between them as regards physical characters and chemical relations has already been given (Art. 453).
The only non-metals present among minerals are carbon, sulphur, and selenium; the last, in one of its allotropic forms, is closely related to the semi-metal tellurium.
The native semi-metals form a distinct group by themselves, since all crystallize in the rhombohedral class of the hexagonal system with a fundamental angle differing only a few degrees from 90°, as shown in the following list:
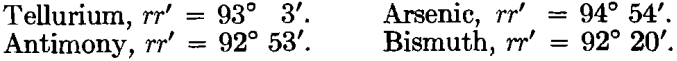
An artificial form of selenium is known with metallic luster and rhombohedral in crystallization, with rr’ = 93°. Zinc (also only artif.) is rhombohedral (rr’ = 93° 46′) and connects the semi-metals to the true metals. Metallic tantalum has been described in cubic crystals.
Among the metals the isometric GOLD GROUP is prominent, including gold, silver, copper, mercury, amalgam (AgHg), and lead.
