Table of Contents
The iron minerals that are at present used as ores are hematite, magnetite, limonite, and siderite; also, occasionally ankerite, goethite, and turgite. Hematite is the most important iron ore.
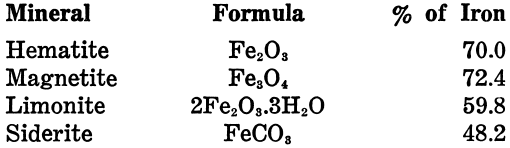
The iron content of the pure minerals is as follows:
Ankerite is a carbonate of lime, magnesia, manganese, and iron. It is of valuable composition, carries only 14 or 15% of iron, and is used more for its lime and magnesia as a flux than for its iron-content. Goethite and turgite come between hematite and limonite in composition, and are found with both, but are comparatively rare.
Iron ores cannot be profitably mined unless they occur in large bodies. The discovery of a few thousand tons, or a vein-like body four or five feet wide, is usually unimportant. To be worth while, the tonnage must be counted in millions of tons.
Impurities in Iron Ores
There are certain impurities that lower the value of iron ores or may even make them valueless. Sulphur in the ore goes partly into the iron and steel and makes them brittle. While it is possible by roasting the ore and by other means to remove the sulphur, the presence of any considerable amount is objectionable. Phosphorus in steel makes it brittle. For steel-making the limit for phosphorus is one hundredth of one per cent for ten per cent of iron in the ore; this is the so-called Bessemer limit. A Bessemer ore carrying 52% of iron must not have more than .052% of phosphorus and the sulphur should not exceed .04%. But since the presence of phosphorus in pig iron increases the fluidity and tends to make sound castings, ores high in phosphorus are used in making foundry pig iron. Titanium is objected to by users of iron ores, because it is said to make a pasty slag and to interfere in other ways with the smooth running of the blast furnace. At present, ores containing more than 5% or 6% of titanic acid (TiO2) are not saleable.
The per cent of iron in the ore as mined must be not less than 50%, if it is to be used without being first improved (beneficiated) by cobbing, washing, calcining, or magnetic concentration. Leaner ore is found in very large quantities; and in a favorable situation, it may be workable by some concentrating process.
Hematite
Hematite is sometimes found in vein-like deposits which are not usually large enough to be important. Such ore-bodies have been found near Kitchener, B.C. and in the Matachewan area, Ontario. Deposits of considerable size have been found at the contacts of igneous intrusions, generally basic, with crystalline limestone, dolomite, and limey shale; the ore is usually of the specular variety, and it is apt to be partly magnetite. The most important hematite deposits are of a sedimentary origin, the ore forming beds in stratified rocks. The hematite beds of the Wabana Mines, Newfoundland, extend for miles under the ocean. They are found in sandstone and shale of Ordovician age. In Nova Scotia, smaller deposits have been found in Devonian and Silurian stratified rocks. The hematite deposits of the Lake Superior region are of sedimentary origin, but the rich ores have undergone natural concentration from the lean ores of the iron formation. These formations are widespread in eastern Canada and the United States; but in only a very small part of the known areas (about 2% in the U.S.A.) have merchantable ores been discovered. The immense iron range in the Labrador peninsula about 400 miles in length has begun to produce high grade hematite from open pits, and in time will rival the famous Mesabi range in Minnesota. At Steep Rock Lake in northwestern Ontario hematite of similarly high grade is being mined from deposits that stand vertical, having been formed by replacement of the rock along a contact of volcanics with crystalline limestone.
Iron Ore Formation
Iron formation consists of iron ore such as siderite, magnetite, and hematite, with silica in the form of chert, jasper, etc., generally in bands, but sometimes not distinctly so. The bands of iron ore are at times high-grade, but are often mixed with a good deal of silica, the whole making an ore too lean for use without concentration. Iron formation is believed to be of sedimentary origin. In Ontario three epochs of deposition are known:
- (a) Keewatin-Grenville,
as in the Michipicoten and Moose Mountain areas: - (b) Timiskamian as in the Porcupine area; and
- (c) Animikean, as in the Thunder Bay area.
There are some expressions in common use in discussing these formations which it will be well to define:
- Jaspilite, iron formation with the quartz layers colored red (jasper) and the rock crystalline, due to metamorphism.
- Ferruginous chert, chert and ore in bands, irregularly mixed, of dark gray, greenish, or reddish color, not plainly crystalline.
- Greenalite, a hydrated silicate of iron of a green color.
- Taconite, another name for ferruginous chert.
Origin of Iron Ore-Bodies
The ore-bodies, consisting of hematite and limonite, with sometimes a little magnetite, have been formed by two distinct processes:
- (a) the weathering of siderite into limonite and hematite, as at the outcrop of the original Helen Mine, Michipicoten area;
- (b) the leaching out of the silica, leaving the concentrated ore in place, as in the Mesabi and other Minnesota ranges and in Labrador.
Only one deposit of the second kind has so far been found in Ontario, that at Loon Lake, east of Port Arthur. In the United States, they have been found mostly in hilly regions and toward the bottom of the slopes. Another common condition is a tight trough, or basin, formed by the iron formation and an intruding dike. Important ore-bodies have been found that were completely without, or almost without, outcrop, in some cases being covered by slate. The hematite is sometimes mixed with enough magnetite to make possible a discovery by means of a magnetic survey.
All varieties of hematite become red or reddish- brown when powdered. Outcrops can be easily “spotted” by striking with a pick or scratching with a knife.
Magnetite
Magnetite is found in three kinds of deposits:
- Those formed by magmatic segregation; these are to be looked for in areas of syenite-porphyry, gabbro, anorthosite, and diorite. Deposits of this class are apt to be high in phosphorus, owing to the presence of apatite. Titaniferous magnetite is found as concentrations from gabbro and anorthosite, sometimes in vein-like bodies, often in irregular masses. Large bodies of titaniferous magnetite are found in Ontario and Quebec. East of Rainy Lake, in Ontario, there is a succession of lenses in a zone of gabbro and anorthosite 14 miles long. In Quebec, near St. Charles, on the Saguenay River, is a large body of titaniferous magnetite in anorthosite.
- Contact metamorphic deposits of magnetite are to be looked for at the contacts of igneous rocks of the intermediate kind (diorite, etc.), or the basic kind (diabase, etc.), with crystalline, limestone, dolomite, or limey shale. The ore bodies are usually irregular and not very large. They are apt to be high in sulphur, from the presence of pyrite and copper pyrites. Garnet, epidote, pyroxene, and apatite are found in the wall rock as well as in the ore. The magnetite deposits of the islands along the Pacific coast, British Columbia, are of this type, as are also many of those of southeastern Ontario and the adjoining part of Quebec, notably the Marmora and Bristol mines.
- Sedimentary deposits are represented by the lean siliceous ore of the iron formation, as at Moose Mountain and Temagami, Ontario, and by magnetic black sand found on the north shore of the St. Lawrence; both of these may prove to be important. They can be concentrated magnetically, and this is being done on a large scale with the taconite of Minnesota, the ore carrying only 25% of iron. The magnetite of the iron mine at Austin Brook, near Bathurst, New Brunswick, is probably a replacement deposit.
Magnetite is easily spotted by its weight, hardness, black powder, and attraction for the magnet.
Limonite
 Limonite, or brown ore, is to be found at the bottom of some bogs and shallow lakes, in favorable situations, where the weathering rocks have yielded iron to the water draining into these basins. The limonite accumulates so fast in some places that a lake bottom may be cropped again after a number of years, as at Radnor Forges, Quebec. Considerable deposits of limonite have been found in places where there is no standing water at present. Limonite is sometimes formed in large quantities by the weathering of the sulphides of iron; such gossan has occasionally been used as an iron ore. At Bannockburn, in the Madoc area, a pyrite deposit was discovered below the limonite, which was being mined as an iron ore; this gossan capping of the pyrite was from eight to fifteen feet deep. In the old iron mines near Londonderry, Nova Scotia, the ore was limonite in fissures originally filled with siderite and other carbonates.
Limonite, or brown ore, is to be found at the bottom of some bogs and shallow lakes, in favorable situations, where the weathering rocks have yielded iron to the water draining into these basins. The limonite accumulates so fast in some places that a lake bottom may be cropped again after a number of years, as at Radnor Forges, Quebec. Considerable deposits of limonite have been found in places where there is no standing water at present. Limonite is sometimes formed in large quantities by the weathering of the sulphides of iron; such gossan has occasionally been used as an iron ore. At Bannockburn, in the Madoc area, a pyrite deposit was discovered below the limonite, which was being mined as an iron ore; this gossan capping of the pyrite was from eight to fifteen feet deep. In the old iron mines near Londonderry, Nova Scotia, the ore was limonite in fissures originally filled with siderite and other carbonates.
Limonite varies a great deal in its appearance. In color, it may be light rusty brown, dark brown, or even black on a fresh fracture. It is sometimes in round grains (shot ore), or in porous round lumps or flat cakes. When ground fine, it forms a yellow powder.
Siderite
Siderite or spathic iron ore, is usually gray or white; but at the surface it weathers to limonite, and the weathering may extend to a considerable depth. A rusty capping may cover a deposit of siderite. Where the ore forms the face of a cliff, the limonite may wash away as fast as it forms, leaving the siderite clean. The ore is found in vast quantities in the iron formation of the Michipicoten District, Ontario; the quantity around the Helen Mine is estimated at not less than 100 million tons; the ore is high in sulphur, owing to the presence of pyrite, and requires to be roasted to remove sulphur as well as to remove carbon dioxide.
