If you need a method for the Electrolytic Determination (Assay) of Copper, use this procedure:
Weigh out sample. In the case on concentrate use .5 grams, with the ore use 1 gram. Decompose slowly with nitric acid on the hot plate using a few crystals of KC103 after the nitrous oxide fumes are expelled. Omit the use of KClO3 on the heads and tails.
Add 5 cc’s H2SO4, heat gently until fuming then fume to dryness. It is necessary that all free H2SO4 be driven off as it will interfere with decomposing. Of copper in the electrolysis.
Add 3 grams of (NH4) SO4, 5 cc’s HNO3 and 25 cc’s water and heat until the soluble salts are in solution. Transfer into the electrolytic beaker copping out with hot water, cool.
After cleaning and weighing the platinum cathode, insert the electrolic circuit as directed. Close the circuit with the solution diluting with water until the level is above the top of the electrodes. Turn on the circuit and regulate at 1.2 amps. Agitate with air at the rate of 100 per minute. Pass the current through for 1 ½ hrs. Add 2 cc’s of HNO3 carry on the electrolysis for another 1 ½ hours.
By following the above directions carefully the copper should now be all precipitated. With the current still flowing lower the solution carefully from the cathode at the same time washing the cathode with water using a steady stream from a wash bottle so that the latter has been thoroughly washed.
Immediately remove the cathode in the beaker of water, rinse in two solutions of alcohol, dry and weigh as soon as possible.
Test the electrolyte for copper by transferring to a 600 cc. Beaker adding 5 cc’s HN03 and boiling down to a bulk of 105 cc’s Cool and add compare with color standards, adding the copper found to the original. Test also the water in the beaker used for the removal of the cathode with H2S.
Treatment of the Cathode
Clean in strong HNO3 and wash well with water, then immerse well in alcohol, ignite and weigh.
Treatment of the Anode.
Iron deposits on the anode and should be removed after each determination with a 10% solution of HCl then washed well with water.
Size of cathode:
5. cm. Diameter and 5 cm. Long
Total immersed in solution – 110 cm.
Size of the anode:
3. cm. Diameter and 5 cm. Long. N.D. 100 a meter reading I.2 amps. Cathode surface immersed 110 cm. Current density – 1.2/110 – 1.09 amps. Electrodes tension. 2.5 volts. Temperature of electrolysis 20-30 C.
Notes on Method
- If copper deposits (darkens) on the cathode shortly after the electrolysis is started and the current is properly adjusted it indicates that all free H2SO4 has not been expelled.
- The presence of ammonia sulphate reduces the resistance of the electrolyte. In determining copper by the electrolytic method in pyrite, using 8 grams of sample, 6 to 8 grams of ammonia sulphate should be used.
- More than 3 hours electrolysis should be avoided unless more HNO3 is added, as the HNO3 is gradually turned to ammonia by the electrolysis.
The separation of copper by means of a current of electricity is largely made use of, and forms the basis of the most satisfactory method for the determination of this metal. If the wire closing an electric circuit be broken, and the two ends immersed in a beaker of acidulated water or solution of any salt, the electricity will pass through the liquid, bringing about some remarkable changes. Hydrogen and the metals will be liberated around that part of the wire connected with the zinc end of the battery, and oxygen, chlorine, and the acid radicals will be set free around the other. Different metals are deposited in this way with varying degrees of ease, and whether or not any particular metal will be deposited depends—(1) on the conditions of the solution as regards acid and other substances present, and (2) on the intensity of the current of electricity used. For analytical purposes the metal should be deposited not only free from the other metals present, but also as a firm coherent film, which may afterwards be manipulated without fear of loss. This is, in the case of copper and many other metals, effected by a simple control of the conditions. It is necessary that the electrodes, or wires which bring the electricity into the solution, should be made of a material to which the deposited metal will adhere, and which will not be attacked by substances originally present or set free in the solution. They are generally made of platinum. There are various arrangements of apparatus used for this purpose, but the following plan and method of working is simple and effective, and has been in daily use with very satisfactory results for the last five or six years.
The battery used is made up of two Daniell cells, coupled up for intensity as shown in fig. 49—that is, with the copper of one connected with the zinc of the other. For eight or ten assays daily the quart size should be used, but for four or five two pint cells will be sufficient.
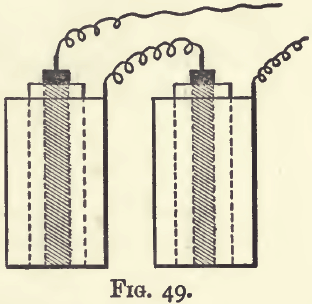
The outer pot of each cell is made of sheet copper, and must be clean and free from solder on the inside. It is provided near the top with a perforated copper shelf in the shape of a ring, into which the inner or porous cell loosely fits. It is charged with a saturated solution of copper sulphate, and crystals of this salt must be added, and always kept in excess. When the battery is at work copper is being deposited on the inner surface of this pot.
The inner or porous pot contains the zinc rod, and is charged with a dilute acid, made by diluting one volume of sulphuric acid up to ten with water. The object of the porous pot is to prevent the mixing of the acid and copper sulphate solutions, without interrupting the flow of electricity. The copper sulphate solution will last for months, but the acid must be emptied out and recharged daily.
The zinc rods must be well amalgamated by rubbing with mercury under dilute acid until they show a uniformly bright surface. They should not produce a brisk effervescence when placed in the acid in the porous pot before coupling up.
The battery when working is apt to become dirty from the “ creeping ” of the copper and zinc sulphate solution. It must be kept away from the working bench, and is best kept in a box on the floor.
The connection of the battery with, and the fixing of, the electrodes may be made by any suitable arrangement, but the following is a very convenient plan. The wire from the zinc is connected by means of a binding screw with a piece of stout copper wire, which, at a distance sufficiently great to allow of easy coupling with the battery, is led along the back of a piece
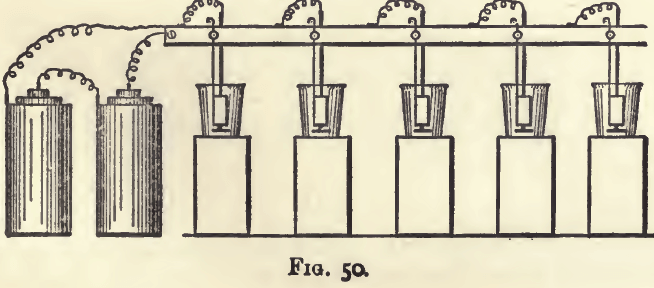
of hard wood. This is fixed horizontally about one foot above the working bench. The general arrangement is shown in
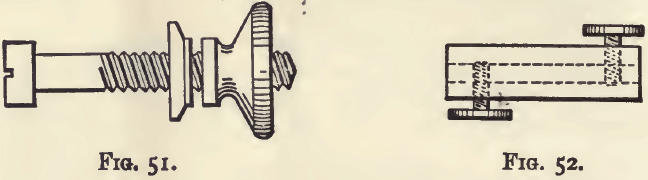
fig. 50, in which, however, for the sake of economy of space, the battery is placed on the working bench instead of on the floor.
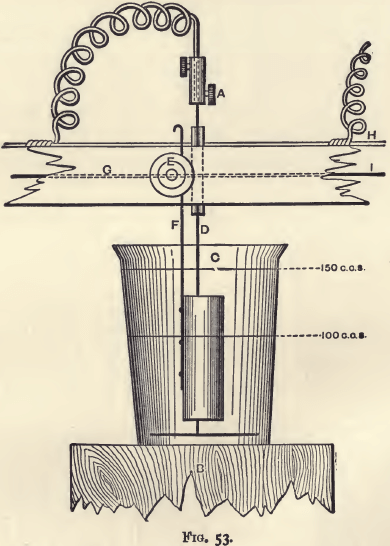
The piece of wood is one inch square and three or four feet long. It is perforated from front to back at distances of six inches by a number of small holes, in which are inserted screws like that shown in fig. 51. These are known as “terminals,” and may be obtained of any electrician. The head of each screw is soldered to the wire mentioned above as running along the back and as being connected with the zinc end of the battery. These terminals serve to fix the electrodes on which the copper is to be deposited. The wire from the copper end of the battery is similarly connected by a connecting screw (fig. 52) with another wire (H in fig. 53), which runs along the top of the rod and has soldered to it, at distances of six inches, cylindrical spirals of copper wire. These should project from the rod at points about half-way between the terminals already described. They may be made by wrapping copper wire around a black-lead pencil for a length of about three inches.
The rod is perforated from top to bottom with a series of small holes, one in advance of each terminal but as near it as possible. Into these short pieces of glass tube are inserted to ensure insulation. These receive the other electrodes, which are connected with the wire leading to the copper end of the battery, through the spirals, with the help of a binding screw. The figure will make this clear. (Fig. 53.)
The electrodes consist of a platinum spiral and cylinder. The spiral should have the shape shown in A, fig. 54. When in work it is passed through one of the holes fitted with glass tubes and connected with the copper end of the battery. The thickness of the wire of which it is made is un-important, provided it is stout enough to keep its form and does not easily bend. The spiral will weigh about 8 grams. The cylinder (C, fig. 54) will weigh about 12 grams. It should have the shape shown in the figure. In working it is clamped to one of the terminals, and on it the copper is deposited. A cylinder will serve for the deposition of from 1 to 1.5 gram of copper. It is made by rivetting a square piece of foil on to a stiff piece of wire, and then bending into shape over a glass tube or piece of rounded wood.
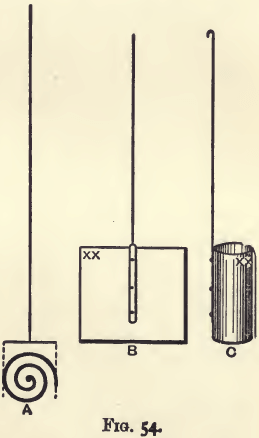
Each cylinder carries a distinctive number, and is marked by impressing Roman numerals on the foil with the blade of a knife. The weight of each is carefully taken and recorded. They lose slightly in weight when in use, but the loss is uniform, and averages half a milligram per month when in daily use. The cylinders are cleaned from deposited copper by dissolving off with nitric acid and washing with water; and from grease by igniting.
The beakers, to contain the solution of copper to be electrolysed, are ordinary tall beakers of about 200 c.c. capacity, and are marked off at 100 c.c. and 150 c.c. They are supported on movable stands, consisting of wooden blocks about six inches high and three inches across. The bar of wood which carries the connecting wires and electrodes is permanently fixed over the working bench, at such a height that, with the beakers resting on these blocks, the electrodes shall be in position for working.
To fix the electrodes to the rod, remove the stand and beaker and pass the long limb of the spiral up through one of the glass tubes. Connect it with the free end of the copper spiral by means of a connecting screw (fig. 52), and then draw out and bend the copper spiral so that the platinum one may hang freely. Screw the wire of the cylinder to the terminal, and, if necessary, bend it so that the cylinder itself may be brought to encircle the rod of the spiral in the manner shown in fig. 53.
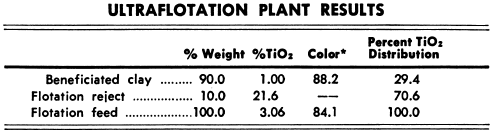
The general method of working is as follows:—The quantity of ore to be taken for an assay varies with the richness of the ore, as is shown in the following table :—

The weighed quantity of ore is dissolved by evaporating with nitric acid and taking up with hydrochloric, as already described. Any coloured residue which may be left is generally organic matter: it is filtered off, calcined, and any copper it contains is estimated colorimetrically. Nearly always, however, the residue is white and sandy. The copper is separated from the solution as sulphide by means of a rapid current of sulphuretted hydrogen. The liquid is decanted off through a filter, the precipitate washed once with hot water and then rinsed back into the flask (the filter paper being opened out) with a jet of water from a wash bottle. Fifteen c.c. of nitric acid are added to the contents of the flask, which are then briskly boiled until the bulk is reduced to less than 10 c.c. The boiling down is carried out in a cupboard free from cold draughts, so as to prevent the condensation of acid and steam in the neck of the flask. Twenty c.c. of water are next added, and the solution is warmed, and filtered into one of the beakers for electrolysis. The filtrate and washings are diluted with water to the 100 c.c. mark, and the solution is then ready for the battery. It must not contain more than 10 per cent, by volume of nitric acid.
The number and weight of the platinum cylinder having been recorded, both electrodes are fixed in position and the wooden block removed from under them. The beaker containing the copper solution is then brought up into its place with one hand, and the block replaced with the other so as to support it. All the assays having been got into position, the connecting wires are joined to the battery. If everything is right bubbles of oxygen at once stream off from the spiral, and the cylinder becomes tarnished by a deposit of copper. If the oxygen comes off but no copper is deposited, it is because the assay solution contains too much nitric acid. If no action whatever takes place, it is because the current is not passing. In this case examine the connections to see that they are clean and secure, and the connecting wires to see that they are not touching each other.
The action is allowed to go on for sixteen or seventeen hours, so that it is best to let the current act over-night. In the morning the solutions will appear colourless, and a slow stream of oxygen will still be coming off from the spiral.
A wash-bottle with cold distilled water and two beakers, one with distilled water and the other with alcohol, are got ready. The block is then removed, the spiral loosened and lowered with the beaker. The cylinder is next detached and washed with a stream of water from the wash-bottle, the washings being added to the original solution.. The current from the battery is not stopped until all the cylinders are washed. After being dipped in the beaker of water and once or twice in that with the alcohol, it is dried in the water-oven for about three minutes, and then weighed. The increase in weight is due to deposited copper. This should be salmon-red in colour, satin-like or crystalline in appearance, and in an even coherent deposit, not removed by rubbing. It is permanent in air when dry, but sulphuretted hydrogen quickly tarnishes it, producing coloured films. With ores containing even very small proportions of bismuth, the deposited copper has a dark grey colour, and when much of this metal is present the copper is coated with a grey shaggy deposit.
It still remains to determine any copper left undeposited in the solution. This does not generally exceed four or five milligrams, and is estimated colorimetrically. Thirty c.c. of dilute ammonia (one of strong ammonia mixed with one of water) are added to the electrolysed solution) which is then diluted up to the 150 c.c. mark with water. It is mixed, using the spiral as stirrer, and, after standing a few minutes to allow the precipitate to settle, 100 c.c. of it are filtered off through a dry filter for the colorimetric determination. Since only two-thirds of the solution are taken for this, the quantity of copper found must be increased by one-half to get the quantity actually present.
The colorimetric determination may be made in the manner described under that head, but where a number of assays are being carried out it is more convenient to have a series of standard phials containing known amounts of copper in ammoniacal solution. By comparing the measured volume of the assay solution with these, the amount of copper present is determined at a glance. These standard bottles, however, can only be economically used where a large number of assays are being made daily.
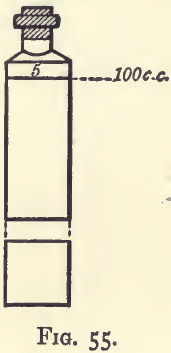
A convenient plan is to get a quantity of white glass four-ounce phials, like that in fig. 55, and to label them so that they shall contain 100 c.c. when filled up to the bottom of the labels. The labels should be rendered permanent by coating with wax, and be marked with numbers indicating the milligrams of copper present. The bottles are stopped with new clean corks, and contain, in addition to the specified quantity of copper, 6 c.c. of nitric acid and 10 c.c. of strong ammonia, with sufficient water to make up the bulk to 100 c.c. The copper is best added by running in the requisite amount of a standard solution of copper, each c.c. of which contains 0.001 gram of the metal.
The standard bottles should be refilled once every three or four months, since their colorimetric value becomes slowly less on keeping. The following determinations of a set which had been in use for three months will illustrate this. The figures indicate milligrams of copper in 100 c.c.: the first raw gives the nominal and the second row the actual colorimetric value of the standards. The difference between the two shows the deterioration.
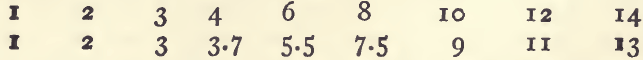
The amount of copper in the assay is got by increasing that found colorimetrically by one-half and adding to that found on the platinum cylinder. The percentage is calculated in the usual way. The following examples will illustrate this, as well as the method of recording the work in the laboratory book :—

In the electrolytic assay of metals, alloys, precipitates, and other bodies rich in copper, the preliminary separation of the copper by sulphuretted hydrogen is unnecessary. It is sufficient to dissolve the weighed sample in 10 c.c. of nitric acid, boil off nitrous fumes, dilute to 100 c.c. with water, and then electrolyse.
General Considerations.—In the preliminary work with the copper sulphide there is a small loss owing to its imperfect removal in washing the filter paper, and another small loss in dissolving in nitric acid owing to the retention of particles in the fused globules of sulphur. To determine its amount the filter-papers and sulphur were collected from forty assays, and the copper in them determined. The average amount of copper in each assay was 0.175 gram; that left on the filter paper was 0.00067 gram; and that retained by the sulphur 0.00003 gram; thus showing an average loss from both sources of 0.00070 gram. The determinations from another lot of forty-two similar assays gave on an average
The loss from these sources is trifling, and need only be considered when great accuracy is required.
The deposition of the copper under the conditions given is satisfactory, but, as already stated, if the solution contain more than 10 per cent, of nitric acid it is not thrown down at all; or if a stronger current is used, say that from three Bunsen cells, it will be precipitated in an arborescent brittle form, ill adapted for weighing. It may be noted here that increasing the size of the cells does not necessarily increase the intensity of the current.
In two determinations on pure electrotype copper the following results were obtained :—
The presence of salts of ammonia, &c., somewhat retards the deposition, but has no other ill effect.
The organic matter generally present in copper ores interferes more especially in the colorimetric determination of the residual copper. It can be detected on dissolving the ore as a light black residue insoluble in nitric acid. It is filtered off at once, or, if only present in small amount, it is carried on in the ordinary process of the assay and separated in the last filtration before electrolysis.
The following experiments were made to test the effect of the presence of salts of foreign metals in the solution during the precipitation of copper by electrolysis:—
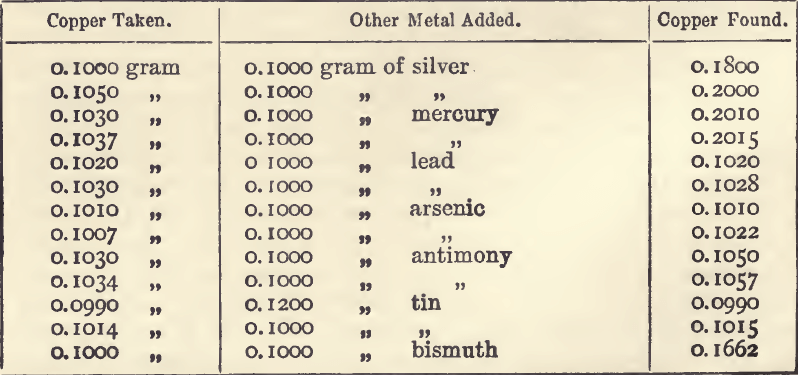
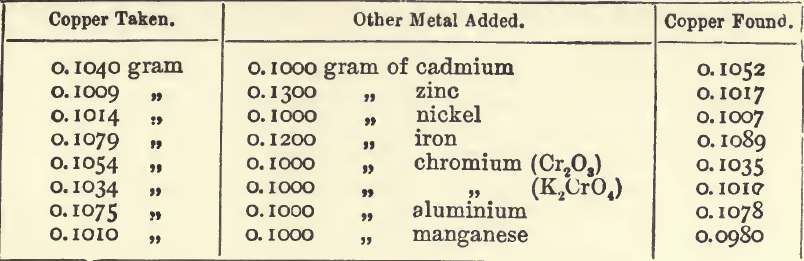
It will be seen from these that mercury, silver, and bismuth are the only metals which are precipitable along with the copper under the conditions of the assay. Mercury, which if present would interfere, is separated because of the insolubility of its sulphide in nitric acid.
Bismuth is precipitated only after the main portion of the copper is thrown down. It renders the copper obviously unsuitable for weighing. It darkens, or forms a greyish coating on, the copper; and this darkening is a delicate test for bismuth. In assaying ores containing about three and a half per cent, of copper, and known to contain bismuth in quantities scarcely detectable in ordinary analysis, the metal deposited was distinctly greyish in colour, and would not be mistaken for pure copper. Ten grams of this impure copper were collected and analysed, with the following results:—

The quantity of copper got in each assay was 0.175 gram, and consequently the bismuth averaged 0.00053 gram.
To separate the bismuth in such a case the deposit is dissolved off by warming it in the original solution. The bismuth is precipitated by the addition of ammonic carbonate, and the solution, after filtering and acidifying with nitric acid, is re-electrolysed.
Determination of Copper in Commercial Copper.—Take from 1 to 1.5 gram, weigh carefully, and transfer to a beaker; add 20 c.c. of water and 10 c.c. of nitric acid; cover with a clock glass, and allow to dissolve with moderate action; boil off nitrous fumes, dilute to 100 c.c., and electrolyse. The cylinder must be carefully weighed, and the electrolysis allowed to proceed for 24 hours. The weight found will be that of the copper and silver. The silver in it must be determined and deducted.
Determination of Copper in Brass, German Silver, or Bronze.—Treat in the same manner as commercial copper. If nickel is present, the few milligrams of copper remaining in the electrolysed solution should be separated with sulphuretted hydrogen, the precipitated sulphide dissolved in nitric acid, and determined colorimetrically.
