Gold is so widely distributed in nature and is so much desired on account of its value that the metallurgy of gold is not only one of the most fascinating but also one of the most important industries. Gold occurs in several forms in nature: as free gold; in combination with sulphur, or in the metallic state associated with iron, copper and lead pyrites and as telluride of gold, Au Te2, silver, arsenic , antimony and zinc are often associated with the various pyrites. While gold seldom occurs except in the presence of silver yet it is not the purpose of this paper to consider silver.
For each of the modes of the occurrence of gold methods have been and are continually being devised. The majority of these processes are unsuccessful, yet a few are recognised as being of intense commercial importance. For all ores not containing gold in the free state chemical methods are necessary. For high grade ores these have been successful, but it is still difficult to extract poor ores profitably unless free milling. The principal processes of commercial importance, and it is only of these we desire to treat, are briefly:
Gold Amalgamation Process
In the amalgamation process the ore is run into a “stamp mill” lined with amalgamated copper plates, the mercury on them dissolving part of the free gold. A stream of water flowing through the stamp carries the ore through a screen placed over the discharge gate of the mortar and then over riffles containing mercury where additional gold is dissolved. Sometimes the tailings are rich enough to cyanide with profit.Often, depending on the character of the ore, the tailings are concentrated, roasted and cyanided. The amalgamation process works well for an ore containing the gold in the metallic state, free from arsenic and much sulphur. To obtain the gold from the amalgam, the mercury is distilled off and used over again while the residual gold is melted.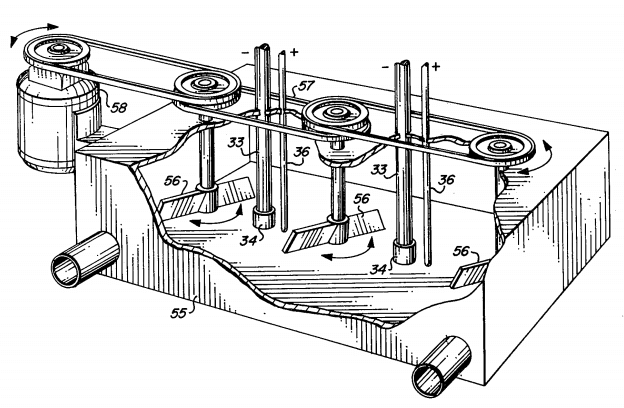
Gold Chlorination
In chlorination the ore is ground, sometimes concentrated, and always roasted. (The tailings are sometimes treated by this process.) The ore is then put into large barrels made of boiler plate lined with lead and fitted with trunnions. Bleaching powder is first put in, then the ore and the requisite amount of dilute sulphuric acid. The barrel is now sealed up and rotated from six to eight hours.
The ore is then flushed out, either into filter presses, or the solution decanted. The gold is precipitated in vats with conical
bottoms either by ferrous sulfate or hydrogen sulfide. The process is much used at Cripple Creek, Colorado. Chlorination is suitable for sulfide and telluride ores or all ores which require roasting. The first cost of the plant is high and the wear and tear of the chlorine gas on the apparatus must be considered.
Cyanide Process
The cyanide process is probably the best method yet devised for the chemical treatment of gold ores. In this process the ore is crushed and ground if necessary and the pulverized ore treated in wooden vats with a potassium or a sodium cyanide solution varying from one half to one per cent in strength. The solution is allowed to act from four to twelve hours. This “strong solution” is filtered off and a solution which was formerly used for washing, run through the ore. This is in turn replaced by a still weaker solution obtained in the same way as the first, and finally with plain cyanide solution. The strongest of these is used in leaching out the next batch of ore and the weaker used for washing out the gold. With filter presses the treatment is complete in ten to twelve hours, while with a leaching process from sixty to seventy hours are consumed. The reactions which are supposed to take place in the cyanide process are as follows. Solution:
2 Au + 4KCN + O + H2O = 2KAuCN2 + 2KOH
Precipitation by means of zinc:
2KAuCN2 + Zn = 2Au + K2ZnCN4
The gold is separated from the solution by either running it over zinc shavings or by means of electrolysis. In either case the gold is deposited as a black mud and is periodically collected and refined. In electrolysis the solution flows continuously through vats containing either lead or tin plate cathodes and oxidized lead anodes. The lead cathodes are cupelled, while with tin plate cathodes the gold is easily scraped off and refined. It may be stated that ores containing silver in large quantities are subject to different treatment.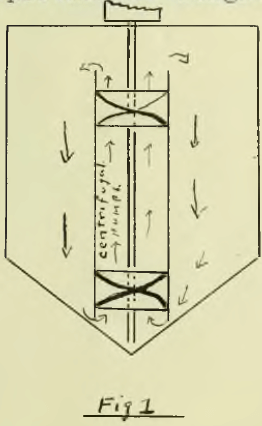
The methods of precipitation from cyanide solutions viz., electrolysis versus zinc is a matter much discussed in the current metallurgical literature. However the electrolytic method seems to be slowly driving out the zinc method and is supplanting the latter in portion of South Africa. The advantages of the cyanide process are, low cost of treatment, small chemical wear and tear, and application to low grade ores. The process is as rapid as chlorination, if filter presses are used. On the other hand the first cost of the plant is high. The process works well on free milling ores in which much of the gold is in a fine state of division and often cyanide is run through the stamp mill. Cyaniding is a profitable method for treating tailings or roasted ores depending on conditions. It may be said at this point that the selection of the best method of treating an ore is one which requires careful study and experiment. Only by the use of a chemical method can all the available gold of an ore be extracted. Of the chemical methods the cyanide process is becoming the favorite though Electrochemists have great faith in the future development of a process employing electricity in extraction. Recently there have been developed two processes employing electricity in the above manner which are the most successful of these yet devised. They are the Hendryx cyanide process and the Greenwalt chlorination process.
In the Hendryx process the ore is crushed to twelve mesh and then placed with the cyanide solution in a vat with a conical bottom fitted with centrifugal mixing apparatus as per figure 1. Electrodes placed near the top of the vat are supposed to deposit the gold as it is extracted. Though well spoken of in the Engineering and Mining Journal it is open to grave doubts except on non-sliming ores free from iron and copper as will be shown later. The Greenwalt process is said to work well but appears to be limited in application. In this process sodium chloride is electrolyzed in diaphragm cells and the resulting chlorine run through the hot ore from the roasters, to chloridize it. Part of the chlorine is used for leaching the ore. It is doubtful if the process can compete with the cyanide except under very favorable conditions. In closing the historical discussion, the writer feels free to say that except as stated, electrical processes have failed signally.
It was the purpose of the writer of this thesis to deal with the best chemical method of treating a sample of ore from Colorado donated by Dr. Howard of Champaign and see to what extent electrical methods were applicable.
Electrolytic Gold Extraction Experiment
Ore from Colorado number “A”. This ore is a “rotten granite” containing a very small amount of sulphide, no telluride, a little copper and considerable iron as oxide. The gold is in a very fine state of division. It was thought that a direct chemical treatment would at least be as good as amalgamation as in all probability amalgamation would necessitate the treatment of tailings. In the first four experiments on chemical extraction it was found that the ore slimed very badly, so that a layer of one eighth inch thickness was almost impervious to water except under pressure. This is one of the main difficulties in treating the ore.
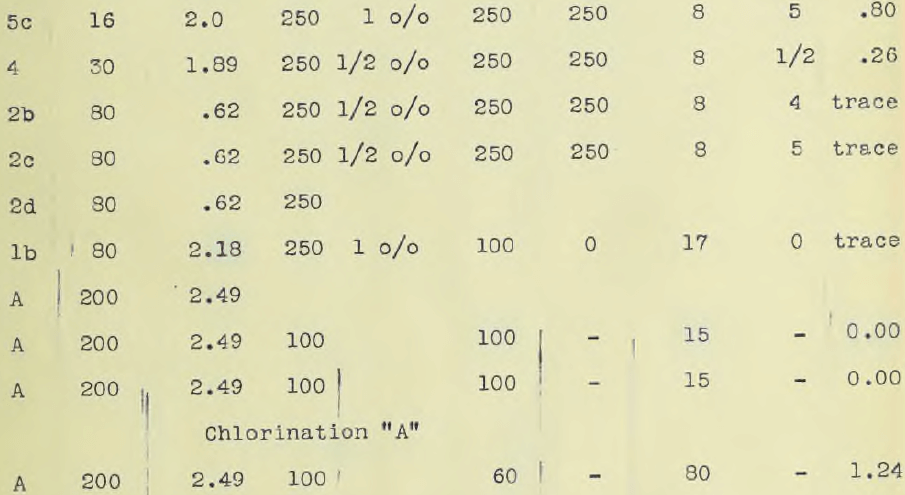
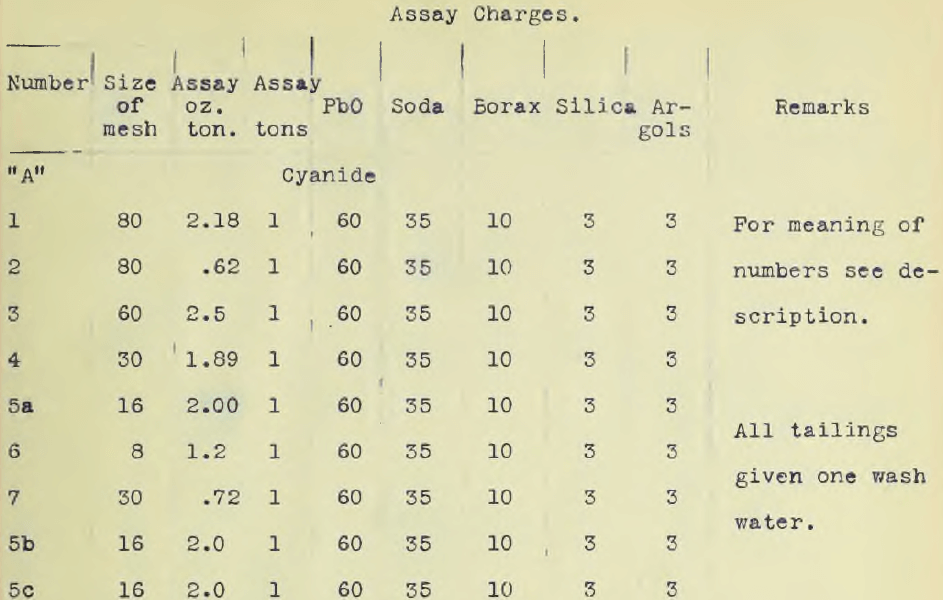
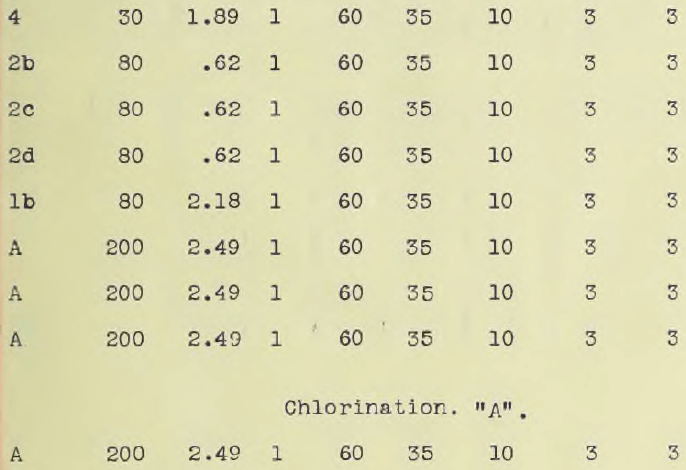
The ore, in all amounting to 100 lbs was crushed to one half inch mesh in a Braun Roller Jaw crusher. Five kilograms were ground by means of a bucking board and a grinding machine to 200 mesh. Ten kilos were crushed to 30 mesh while ten kilos were sifted and assorted as it came from the crusher set at one half inch mesh. About two kilos passed an 80 mesh sieve, one half kilo a 60 mesh, ½ kilo a 30 mesh, ½ kilo a 16 mesh and a kilo an 8 mesh while the remainder was one half inch mesh. Part of this was ground after sampling to a 100 mesh and designated ore number “A2”. The object of classifying the ore according to size as delivered from the crusher was first, to obtain ore of different sizes so that the minimum size for rapid treatment could be readily ascertained; second, to see if the “fines” carried much of the values. The different sizes were assayed, the results of which are given in the accompanying table.
Stirring Apparatus
It was thought necessary to thoroughly agitation the ore during extraction and a stirring apparatus was constructed of glass rod, tube and wood as shown in sketch 3.
Using Electricity as a Method of Gold Extraction
One hundred grams of 100 mesh ore were placed in a jar with 500 grams of water and 15 grams of sodium chloride. This was electrolyzed with a current of 0.3 – 0.6 ampere for three hours, current density 0.1 – 0.2. The electrodes were of platinum and the solution was slowly agitated. The ore then stood twelve hours. At the end of that time the solution was filtered off and electrolyzed for gold. A mere trace of gold was obtained. This was repeated several times varying the current density up to one ampere per square cm. No better results were obtained. One hundred grams of ore were now treated, by slowly agitating, with one hundred grams of 15 per cent sodium chloride solution. A spongy black precipitate was obtained on the cathode by electrolysis which flaked off and fell back into the solution. The experiment was repeated several times varying the current density from 0.1 ampere per square cm. up to 1.0 ampere per square cm. with the results given above. The current was now increased to 3 amperes per square cm. with the purpose of generating chlorine. After six hours the current was turned off and the solution allowed to stand for twelve hours. On filtering the solution and electrolyzing for gold, the precipitation was incomplete. The precipitate contained much iron and copper, was black and non-adherent. Rotating cathodes and heat were tried with no better success in precipitating. A rotating cathode was used on 100 grams of ore and 100 grams of 15 per cent sodium chloride solution. No deposit was obtained. On filtering off the solution and electrolyzing, results were similar to those given above. In all these experiments platinum electrodes were used. Using the solutions given below on separate portions of 100 grams of ore experiments were conducted in the same manner as those just described in an endeavor to increase the solubility of the gold. Slow agitation was used. The solutions employed were :
15 per cent sodium chloride 100 c.c., calcium fluoride 2 grams;
15 per cent sodium chloride 100 c.c., potassium bromide 2 grams;
15 per cent sodium chloride 100 c.c., chromic acid 2 grams;
15 per cent sodium chloride 100 c.c., potassium cyanide 2 grams;
1 per cent potassium cyanide 100 c.c.;
½per cent potassium cyanide 100 c.c.;
5 per cent potassium cyanide 100 c.c.
The deposits obtained, using simultaneous extraction and precipitation, were black and spongy. They contained much iron, sand and clay. The deposits from the different solutions did not differ in character and were all unsatisfactory. On filtering the solution from the ore it was found that electricity had not aided cyanide extraction nor given a good extraction with the other solutions. Apparently electrolytic extraction tended to aid the solution of undesirable substances. A porous pot was placed in a jar containing dilute sulphuric acid and a large sheet lead cathode. In the porous pot were placed a carbon anode, 100 grams of ore and 50 c.c. of a saturated sodium chloride solution and 5 grams of sodium chloride. A current of 3 amperes was passed for 3 days, giving copious evolution of chlorine. An assay of the tailings after washing showed an extraction of 50 per cent. (See table.) Using cyanide solutions a large number of experiments were carried on with electrodes of carbon and lead, Acheson graphite and lead, lead and oxidized lead, and all platinum. Lead cathodes and oxidized lead anodes gave the good results. The extraction from the ores on filtering indicated that the current had aided in the solution of iron and copper to such an extent that precipitation was difficult. The writer believes that a solution which will not give good electrical precipitation will not give a good precipitation of any kind. Carbon and graphite are unsuitable electrode materials as they disintegrate. In electrolyzing the ore pulp much of the slime was deposited on the cathode. On account of the difficulties encountered electrical extraction was given up though it was determined to continue electrical precipitation experiments. As the experiment on chlorination using the porous pot had failed and as the cyanide solutions had given good extraction, it was determined to study the process more in detail.
During all the experiments above the filtering of the ore had given great trouble and attention was now turned to this. One hundred grams of ore and 100 grams of solution gave a very fine mud, hence settling was impossible. The ore slimed so badly that leaching would not work. Centrifugal separation was incomplete. The mixture of 100 grams of 100 mesh ore was placed in bottles on a board 18 inches in diameter and rotated rapidly, but the separation was unsatisfactory. Twelve hours were required to filter 100 grams by suction.
The Chamberlain filter would filter 100 grams of 200 mesh ore in one hour. A drawing of this filter is appended. Ninety per cent of the solution could be recovered by this method.(Note. By mixing equal parts of ore “A” with another associated with it which were separately ground to 100 mesh, rapid leaching was possible. The mixed ore assayed $8.25 per ton.) By the use of the cyanide method the maximum rapidity of extraction was found for different mesh with use of rapid agitation.
The accompanying table gives these results. Ore down to and including 30 mesh gave a complete extraction with solutions ranging from one half to one per cent in eight hours including washing. The accompanying table also gives the assay charges. Complete precipitation was obtained at room temperatures using a rotating cathode of platinum. (See photograph.) The deposit was of a bright metallic appearance and precipitation was complete in two and one half hours using current densities varying from 0.05 ampere to 0.25 per sq. cm. The solution must be made alkaline. A rotating oxidized lead anode, prepared in a one per cent potassium permanganate solution by electrolysis, and lead cathode gave good precipitation but platinum was more convenient. It was hoped to obtain the gold as a sheet so as to obviate refining and allow the gold to be taken out direct, but time did not permit.
The use of electrical precipitation in preference to zinc was adopted as the ore was located in inaccessible regions. For such localities a proper method of electrical precipitation ought to offer the following advantages: case of refining, little trouble to maintain through difficulties in getting supplies and the possibility of obtaining the gold at shorter periods of time than is possible with zinc. While this was not accomplished, it was the hope of the writer to do so.

The tailings gave a mere trace of gold. By the ordinary balance, only weighing to 0.2 m/g, the difference between the weight of the gold obtained and by assay was 0.12 oz. per ton which amounted to an extraction of 80 %, of gold present, chemical tests did not show even a trace of gold in the electrolyte.
Concluding the Electrolytic Extraction of Gold
The experimental results obtained in this thesis tend to show:
- That by agitating a weak cyanide solution a rapid and complete extraction can be obtained from the ore at hand ;
- That an efficient stirring apparatus could be easily designed ;
- That 30 mesh was sufficiently fine for complete extraction in a short period of time ;
- That the “fines” carried the highest values ;
- That the Chamberlain filter solved the slime problem in that 90 per cent of the liquid could be recovered rapidly, and for this reason the writer is in favor of filter pressing and can find support for this statement in reference 11;
- That combined electrolytic extraction and precipitation with either chlorination or cyaniding was unsuccessful;
- That separate electrical extraction was unsuccessful for either process ;
- That with this ore cyanidation was easily adapted to electrical precipitation and that the gold could be completely obtained from alkaline solutions as a bright metallic deposit by means of a rotating cathode at ordinary temperatures.
- From the article of F. L. Bosqui, on the treatment of a much more refractory ore (See ref.11), it is reasonable to conclude that ore “A” can be treated at a cost and leaving a profit. This supposes a 100 ton plant.
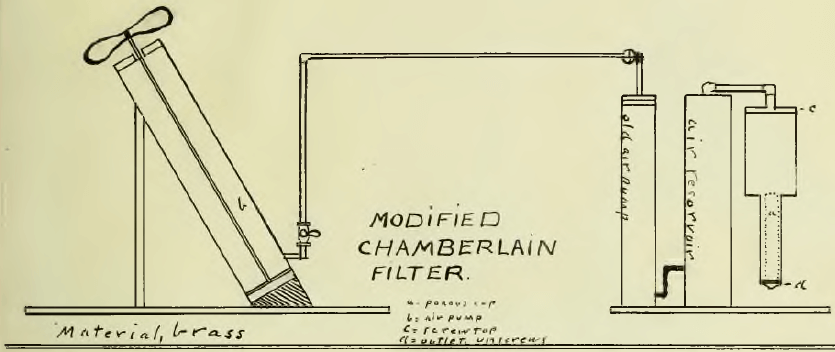 Chamberlain Filter
Chamberlain Filter
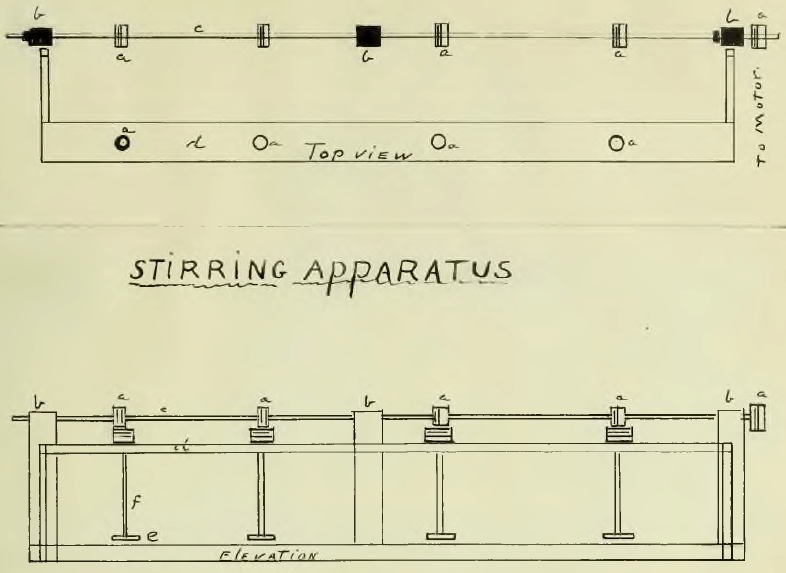 Stirring Apparatus
Stirring Apparatus
Reference: The extraction of gold ore : with reference to electrolytic methods.
