The study and use of ferrocyanides was initiated with the discovery of the pigment “Prussian Blue” by Diesbach in 1704. Thus, they are among the earliest commercial chemicals and have been produced in large quantities for many years. In the United States, American Cyanamid Company, with its ample supplies of cyanides as starting materials, has been the principal manufacturer of ferrocyanides.
Because of their use in the preparation of pigments and because of their unique properties, the ferrocyanides have been the subject of many investigations, and a large amount of information on them is available in the scientific literature. This book presents a summary of the known physical and chemical properties of sodium and potassium ferrocyanides and of hydroferrocyanic acid together with known and proposed applications. The field of pigments is so broad and so complex that a major part of the literature on ferrocyanides has been devoted to it. The purpose of this book, therefore, is to emphasize many of the applications that have been neglected previously. It is felt that the information presented in this review will be of value not only to a variety of different industries, but also to scientists in schools and colleges. The properties of the alkali ferrocyanides and derivatives easily obtained from them are of particular interest in chemical synthesis, separation and purification of commercial chemicals, steel processing, mineral dressing, and photography. We invite correspondence concerning sodium ferrocyanide, potassium ferrocyanide, and their derivatives.
Ferrocyanides Synthesis
The chemistry of the ferrocyanides dates back more than two hundred years to 1704 when Diesbach, a color maker in Berlin, accidentally discovered Prussian Blue (potassium ferric ferrocyanide). Credit for the first application of Prussian Blue as a dye, however, belongs to Dippel.
 Macquer, in 1753, showed that Prussian Blue could be treated with potash to form a soluble, yellow potassium salt. Caustic produced a similar sodium salt, and the association of the latter salts with Prussian Blue, as well as their color, led to the familiar names “Yellow Prussiate of Soda” and “Yellow Prussiate of Potash”.
Macquer, in 1753, showed that Prussian Blue could be treated with potash to form a soluble, yellow potassium salt. Caustic produced a similar sodium salt, and the association of the latter salts with Prussian Blue, as well as their color, led to the familiar names “Yellow Prussiate of Soda” and “Yellow Prussiate of Potash”.
Macquer demonstrated that the potassium salt formed from Prussian Blue was identical to yellow blutlaugensalz, a compound then being prepared by leaching the mass obtained from the fusion of animal residues with potash and iron. This was the first synthetic method for producing potassium ferrocyanide.
HOW TO MAKE A FERROCYANIDE
Sodium and potassium ferrocyanides may be synthesized by two general methods. The first involves fusion of iron or its compounds with metallic cyanides, and the second is the double decomposition of other metallic ferrocyanides with sodium or potassium compounds.
The preparation of yellow blutlaugensalz, as originally carried out by the fusion of potash with iron compounds and animal residues such as hide, horns, dried blood, leather, etc., is an example of the first method.
K2CO3 + Nitrogenous Matter → KCN + ….
6 KCN + Fe++ → K4Fe(CN)6 + 2 K+
The fusion of alkali with peat, carbazole, carbon in the presence of nitrogen, calcium cyanamide, and other nitrogen-containing organic compounds, together with iron or its compounds can lead to similar results. If aqueous solutions of sodium or potassium cyanide are boiled with finely divided iron compounds, the respective ferrocyanides also are produced.
Likewise, these salts are formed when alkaline aqueous suspensions of ferrous sulfide, ferrous carbonate, and other ferrous or ferric salts are combined with hydrogen cyanide or with ammonium cyanide.
2 Na2CO3 + FeS + 6 HCN → Na4Fe(CN)6 + H2S + 2 H2O + 2 CO2
6 NH4CN + 4 KOH + Fe(OH)2 → K4Fe(CN)6 + 6 H2O + 6 NH3
Alkali thiocyanates can be desulfurized by fusion with iron-zinc alloys to form alkali cyanides, which then react in the usual manner.
Several processes, some of which are still important in Europe, illustrate the second general method of synthesis. Ferrocyanides of calcium, magnesium, ammonium, and iron, obtained directly or indirectly during the absorption of hydrogen cyanide from coal gas, have been treated with alkali, sylvinite (a mineral containing sodium and potassium chlorides), sodium sulfate, or sodium chloride, to yield sodium and potassium ferrocyanides.
Minor amounts of ferrocyanides have been prepared commercially by the alternating current electrolysis of potassium cyanide with iron electrodes. During the process, anodic solution of the iron occurs.
6 KCN + Fe + 2 H2O → K4Fe(CN)6 + 2 KOH + H2
Sodium and potassium ferrocyanides can be prepared by the reduction of the respective ferricyanides. Potassium ferricarbonylpentacyanide, K3Fe(CN)5CO, is converted to potassium ferrocyanide when heated with powdered iron.
COMMERCIAL PRODUCTION of Ferrocyanides
Sodium Ferrocyanide
Crude sodium cyanide containing some calcium salts, soda ash, and ferrous sulfate is heated in aqueous solution to form the soluble sodium and calcium ferrocyanides. During the reaction, calcium carbonate and calcium sulfate precipitate. The calcium ferrocyanide is decomposed by the addition of more sodium carbonate, and the slurry is filtered. The filtrate is concentrated, and the sodium ferrocyanide crystallizes as the decahydrate, Na4Fe(CN)6·10H2O.
Potassium Ferrocyanide
Crude mixtures of sodium and calcium cyanides are heated with ferrous sulfate in solution to give sodium and calcium ferrocyanides. Potassium chloride is added to the solution to produce the nearly insoluble double salt, potassium calcium ferrocyanide, which is collected by filtration. This is slurried and heated with an aqueous solution of potassium carbonate, which effects the precipitation of calcium carbonate and forms potassium ferrocyanide. From the settled and filtered solution, the product crystallizes as the trihydrate, K4Fe(CN)6·3H2O. The following indicate the various reactions involved:
6 NaCN + FeSO4 → Na4Fe(CN)6 + Na2SO4
3 Ca(CN)2 + FeSO4 → Ca2Fe(CN)6 + CaSO4↓
Na4Fe(CN)6 + Ca2Fe(CN)6 + 4 KCl → 2 K2CaFe(CN)6↓+ 4 NaCl
K2CaFe(CN)6 + K2CO3 → K4Fe(CN)6 + CaCO3↓
Physical Properties
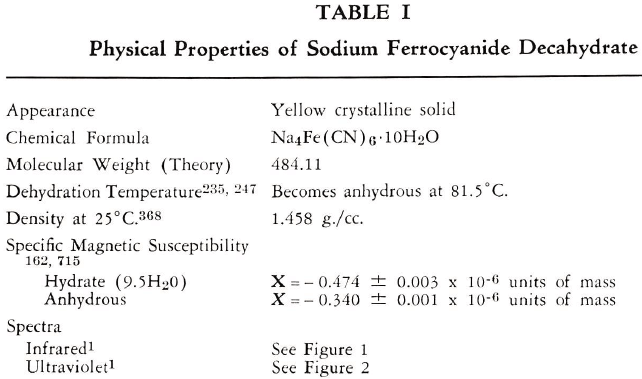
SODIUM FERROCYANIDE
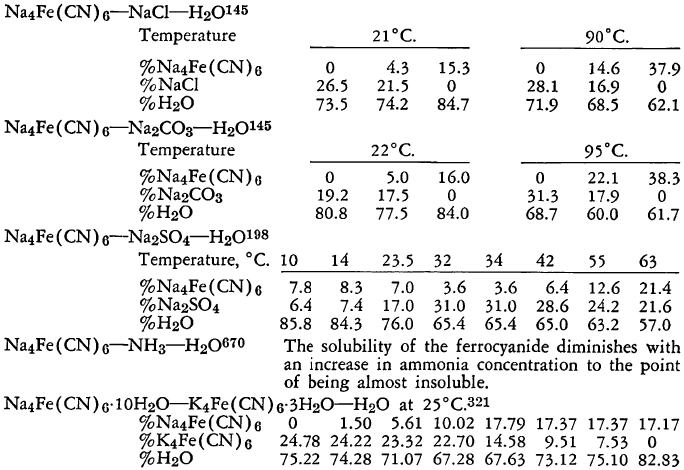
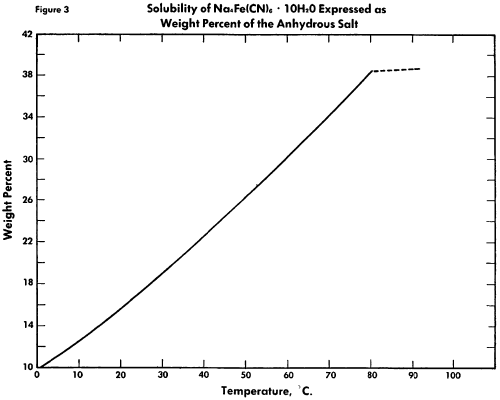
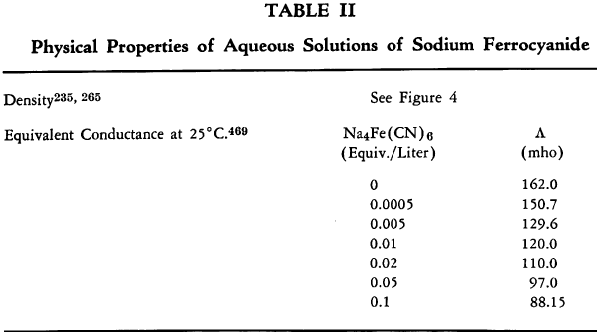
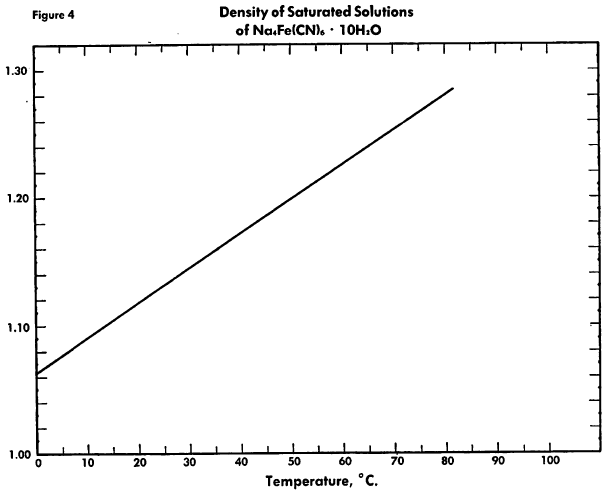
The known physical properties of solid sodium ferrocyanide decahydrate are outlined in Table I and Figures 1-3. The properties of aqueous solutions of anhydrous sodium ferrocyanide appear in Table II and Figure 4.
Crystallography
Crystal System Monoclinic
Crystal Class Prismatic
Axial Ratio a:b:c = 0.8515:1:0.7867 β = 82°26′
Crystal Forms {010} {110} {011} {100} {101}
Crystal Habit Columnar
Cleavage {010} indistinct
Optical Properties
Refractive Indexes Nx = 1.5193, Ny = 1.5295, Nz = 1.5436
Birefringence 0.0243
Optic Sign (+)
Optic Axial Angles 2V 81°25′
Dispersion Weak
Orientation O.A.P. {010} ZAC + 74°
Pleochroism Y>X>Z
Solubility in Water
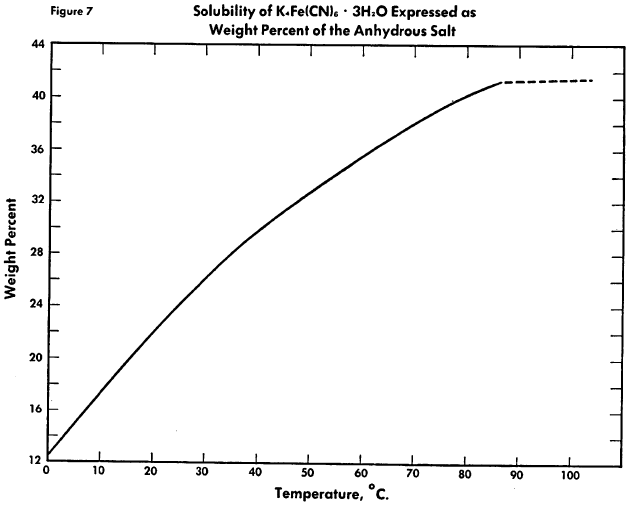
Binary System See Figure 3. Investigations have been carried out over a temperature range of 0-95°C. A eutectic for the binary system of the decahydrate and anhydrous salt has been observed at 81.5°C.
Ternary Systems
Quaternary Systems at 25°C.
Na4Fe(CN)6—Na2CO3—Na2SO4—H2O
Na4Fe(CN)6—Na2CO3—NaCl—H2O
Na4Fe(CN)6—Na2SO4—NaCl—H2O
Solubility in Organic Solvents
Essentially insoluble in most organic solvents.
POTASSIUM FERROCYANIDE
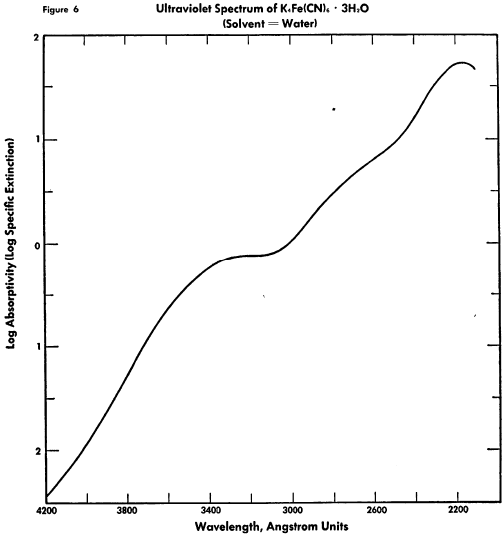
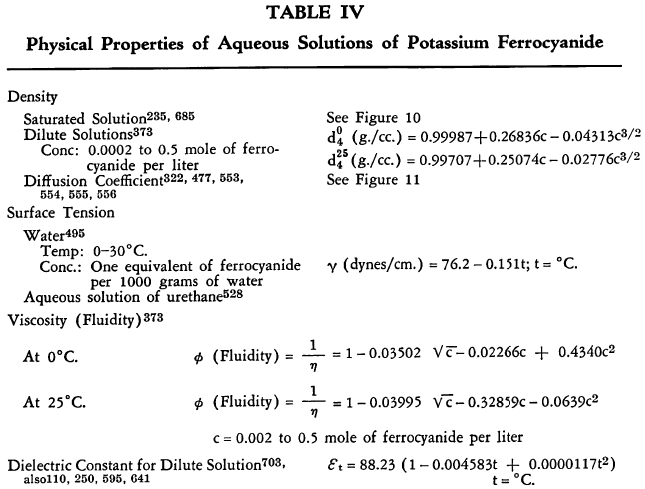
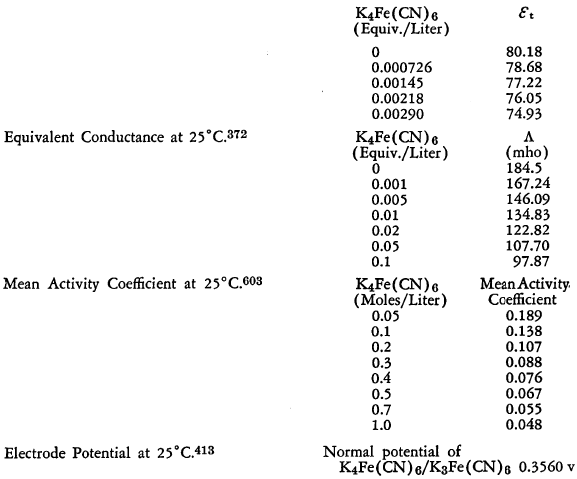
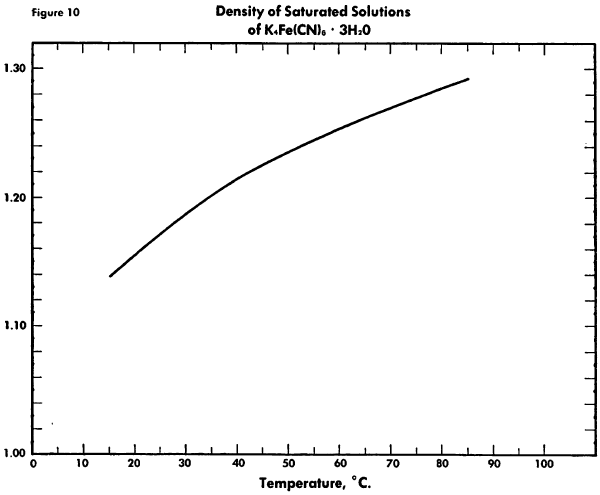
The known physical properties of potassium ferrocyanide trihydrate are outlined in Table III and Figures 5-9. The properties of aqueous solutions of anhydrous potassium ferrocyanide appear in Table IV and Figures 10-11.
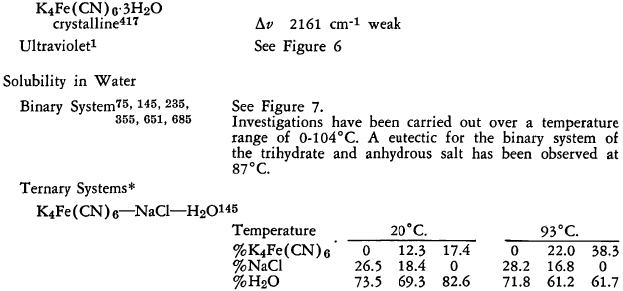

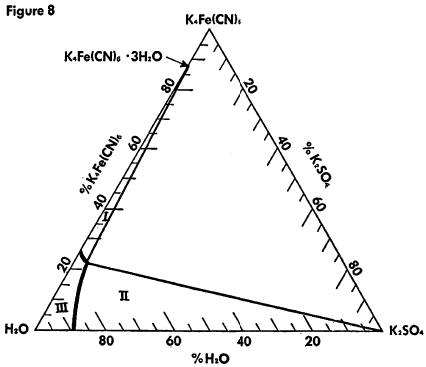
Ternary System K4Fe(CN)6 —K2SO4 —H2O at 30°C
Region I—K4Fe(CN)6·3H2O and solution
Region II—K2SO4 and solution
Region III —Unsaturated solution
Concentrations are in % by weight
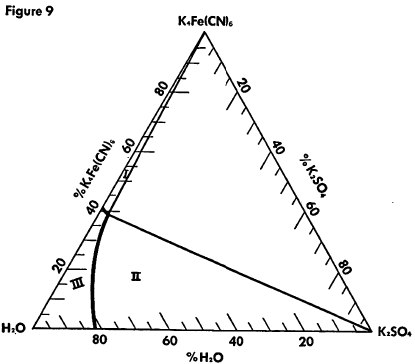
Ternary System K4Fe(CN)6—K2SO4—H2O at 90°C.
Region I—K4Fe(CN)6 and solution
Region II—K2SO4 and solution
Region III —Unsaturated solution
Concentrations are in % by weight
STRUCTURE OF THE FERROCYANIDES
As early as 1795 it was realized by most workers that the structure of even the simple ferrocyanides was extremely complex. Berthollet pointed out that the cyanide groups in these salts were chemically unreactive. Proust and Porrett added the observations that the iron in the ferrocyanides is tightly bound and cannot be precipitated with sulfides, is not removed by electrolysis, and is not so easily oxidized as in most ferrous salts. The correct composition of hydroferrocyanic acid, H4C6N6Fe, was first reported by Berzelius, and later workers verified that the acid is tetrabasic.
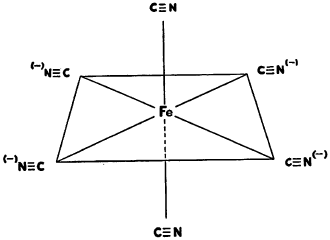
Numerous structures of potassium ferrocyanide and hydroferrocyanic acid have been proposed in attempts to account for all their properties. Some of the early proposals are pictured below:
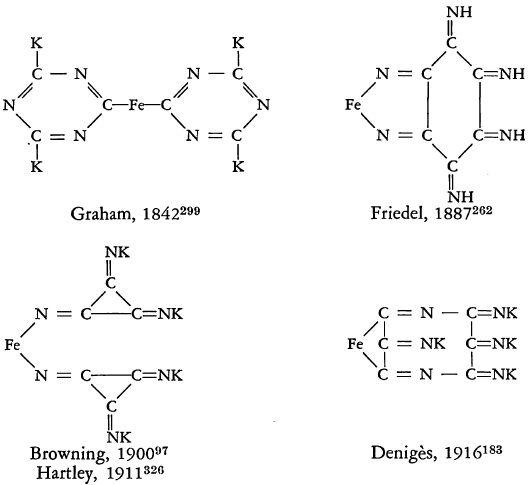
All of these structural formulae, however, fail to account for the replaceability of only one cyanide group by carbon monoxide, nitric oxide, or water. At one time Briggs reported that two isomers of potassium ferrocyanide could be obtained. This was shown to be erroneous by Bennett, and later Briggs was able to demonstrate that one of the isomers actually consisted of mixed crystals of potassium ferrocyanide and potassium ferroaquapentacyanide. This tendency to form mixed crystals has been partially responsible for much of the confusion in early investigations.
Werner’s theory regarding co-ordination complexes provides the most reasonable structure for the ferrocyanide ions as follows:
Pauling has accounted for a structure of this nature on the basis of resonance, making the assumption that the iron-carbon bonds are resonance hybrids made up of the following:
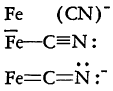
These range from a pure electrostatic contribution to that of a doubly covalent bond that utilizes another third orbital of the iron atom. For the whole ion, therefore, a structure such as that shown below may be written:
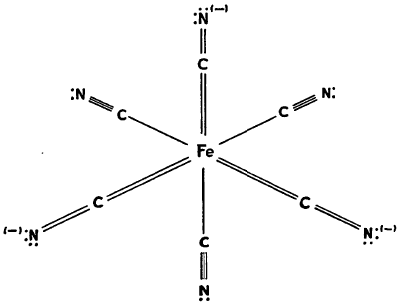
The bonds, of course, may be redistributed among the various atoms giving at least six combinations and thus, considerable resonance stabilization. It can be seen that since each cyanide group is essentially equivalent, the replacement of any one by nitrous oxide or carbon monoxide would not be unreasonable. The latter groups are able to contribute in a similar manner to the over-all resonance picture as, for example, in iron enneacarbonyl, Fe2(CO)9.
Absolute proof of the structure of the ferrocyanides has not yet been offered, but most workers now seem to feel that the postulates of Werner and Pauling are reasonable. A source of controversy that still exists, however, rests in whether some or all of the cyanide groups are in the isocyanide form.
In order to clarify later references to some of the complex iron ferrocyanides, a brief outline of their structural complexities is offered here. For a more complete discussion, the reader is referred to other sources.
As noted earlier, the Iron Blues have been known since the early eighteenth century, and various trivial names have been given to them such as Prussian Blue, Turnbull’s Blue, and Berlin Green. Strict chemical formulae cannot be written for many of them because their composition is indefinite. For example, the most generally accepted formula for “insoluble” Prussian Blue is that of ferric ferrocyanide, Fe4[Fe(CN)6]3, and for “soluble” Prussian Blue is potassium ferric ferrocyanide, KFeFe(CN)6. The “soluble” Prussian Blue may contain sodium, ammonium, lithium, or water instead of potassium. It does not actually dissolve in water, however, but forms colloidal solutions. Turnbull’s Blue is supposedly ferrous ferricyanide, Fe3[Fe(CN)6]2. However, x-ray data indicate that it is not distinguishable from ferric ferrocyanide or “insoluble” Prussian Blue. Berlin Green, on the other hand, is ferric ferricyanide FeFe(CN)6.
Investigations into the structure of these pigments has been extensive, and most of the tools at the chemist’s disposal such as x-rays, magnetic susceptibility measurements, absorption spectroscopy, and chemical analysis have been utilized. Hampering these investigations, however, have been such phenomena as colloid and gel formation and changes in the structures during analysis due to oxidation, reduction, or variation in pH.
Recent x-ray measurements have indicated that the crystals of “soluble” Prussian Blue are cubic. One-eighth of a unit cell appears roughly as follows:
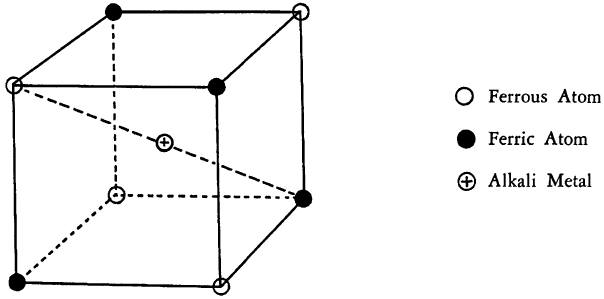
Each cube contains alternately a ferrous or ferric iron atom in each of the corners, and the cyanide groups lie in the edges of the lattice. The alkali atoms, if present, appear in the center of alternate cubes and may be replaced by ammonium ions or water.
If one considers the following equilibrium it becomes easy to understand how difficult it is to locate the specific atoms in the crystals:
![]()
Undoubtedly the position of this equilibrium has some effect on the color of the crystals as well as on their actual structure; and if a foreign atom or molecule is adsorbed on, or actually incorporated into, the crystal lattice, other changes occur. It is not surprising, therefore, that in the following chapters reference to specific complex ferrocyanides must be somewhat vague and that in all cases the specific conditions for their preparation are of utmost importance.
Ferrocyanide Chemical Properties

SODIUM AND POTASSIUM FERROCYANIDES
The reactions of sodium and potassium ferrocyanides discussed in this section are those of the dry salts, either as solids or in essentially anhydrous media. Reactions involving the ferrocyanide ion or free hydroferrocyanic acid are to be found in subsequent sections.
Sodium ferrocyanide may be prepared as a white, anhydrous, nonhygroscopic powder, or, more commonly, as a pale yellow, crystalline decahydrate. In spite of earlier reports to the contrary, work late in the nineteenth century established that the only hydrated form was that containing ten moles of water.
Anhydrous potassium ferrocyanide is a white powder and is extremely hygroscopic. When exposed to ultraviolet light, it gradually turns blue, probably due to the formation of Prussian Blue on the crystals.
Potassium ferrocyanide is usually prepared as its yellow, crystalline trihydrate. Again, only one hydrated form is known. This product is stable in air and laboratory light for at least a year, but in direct sunshine, the color may change very slowly to a greenish-blue. If the crystals are damp, exposure to light appears to cause some slow liberation of hydrogen cyanide with the formation of a fine coating of ferric hydroxide on their surfaces.
Due to the greater availability of potash to many of the early workers in Europe, most of the reactions outlined below were carried out using potassium ferrocyanide. It may be expected, however, that the sodium salt will behave in essentially the same manner in most cases.
Dehydration
At atmospheric pressure, room temperature, and when the relative humidity is low, sodium ferrocyanide decahydrate is very slightly efflorescent. Steady dehydration does not begin, however, until the temperature is raised above 50 °C. If the crystals are irradiated by ultraviolet light, efflorescence occurs more easily, particularly at the crystal edges. For example, at 47°C., irradiated crystals of sodium ferrocyanide lose 3.5 times as much water in two and a half hours as do those which have not been irradiated. As might be expected, dehydration also occurs readily at room temperature under substantially reduced pressure.
The dehydration of potassium ferrocyanide trihydrate has been studied in somewhat greater detail than that of the sodium salt. All the water is lost at room temperature when the pressure is below 3 mm., and under these conditions, drying agents such as sulfuric acid or calcium chloride are useful. It is reported that in a carbon dioxide-free airstream, the trihydrate loses one mole of water at 30°C. and two at 40°C. in six to seven hours. One author reports, however, that the last one-half mole of water is difficult to remove at this temperature. Quantitative dehydration at atmospheric pressure takes place much more rapidly at 80-95 °C. with essentially no decomposition. The following equation has been developed for the vapor pressure of water in millimeters over the salt at 19-100°C. and atmospheric pressure:
log P H2O = — 2610.1539/T + 9.8782
Removal of at least some of the water by the reaction of the trihydrate with calcium carbide, phosphorus trichloride, or acetyl chloride recently has been reported. Vanadium oxytrichloride also is said to remove water from hydrated potassium ferrocyanide.
Irradiation
As noted above, irradiation with ultraviolet light causes some decomposition at the edges of the crystals of sodium ferrocyanide making them generally more reactive.
When crystals of potassium ferrocyanide are subjected to the prolonged action of x-rays, decomposition and a change in color are noted.
Pyrolysis
Anhydrous sodium ferrocyanide starts to decompose in vacuo at 435 °C. giving as products a mixture of sodium cyanide, iron, carbon, and nitrogen.
![]()
At temperatures above 400 °C. potassium ferrocyanide may be pyrolyzed with decomposition according to the following general equation:
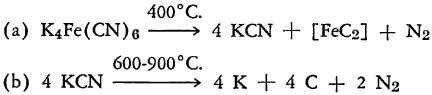
The iron carbide noted in brackets actually is not formed, but there is produced a reactive residue consisting of graphite, alpha-iron, and iron carbide, Fe3C which occur in the stoichiometric proportions for the formula recorded.
Depending upon temperature, pressure, and whether an oxidizing or reducing atmosphere is present, the pyrolysis leads to different end products. Whereas reaction (b) starts at a satisfactory rate only at pressures near 1 mm. Hg, slowing down rapidly as the pressure is raised to 100 mm. Hg, reaction (a) is said not to be influenced by pressure up to one atmosphere.
At moderate temperature in vacuo, dipotassium ferrous ferrocyanide has been noted.
2 K4Fe(CN)6 → K2FeFe(CN)6 + 6 KCN
At red heat, however, this product undergoes further decomposition.
K2FeFe(CN)6 → 2 Fe + 2 KCN + 2 (CN)2
If air is admitted to the hot pyrolyzate, various iron oxides as well as potassium cyanate may be produced.
Pyrolysis at 350°C. and under 200 atmospheres of hydrogen and nitrogen also yields a highly reactive form of iron. The latter is catalytic for the conversion of hydrogen and nitrogen into ammonia at high pressure and 430°C.
When a mixture of sodium and potassium ferrocyanides is heated at temperatures above 600 °C., a sodium-potassium alloy, liquid at room temperature, is said to form in silvery droplets. This apparently results from further decomposition of the sodium and potassium cyanides produced from the ferrocyanides.
Reaction with Water Vapor
Sodium ferrocyanide is decomposed very slowly by high pressure steam with the evolution of ammonia and the formation of formates. At 300 lbs. of steam pressure and temperatures near 250 °C., a yield of ammonia corresponding to 46.6 percent of theory was obtained in four and three-quarter hours. Addition of caustic or lime to the ferrocyanide increases slightly the rate of evolution of ammonia.
Reaction with Ammonia
If hydrated potassium ferrocyanide stands in an atmosphere of cold ammonia, a reddish-brown coating develops on it. An increase in the temperature, however, causes the crystals to become bluish. The anhydrous ferrocyanide, on the other hand, neither absorbs ammonia nor reacts with it at room temperature.
Oxidation
Oxidation of anhydrous potassium ferrocyanide has been carried out by pyrolysis of the salt in an atmosphere of oxygen at 300 °C. and 400 °C. with the results given in Table V.
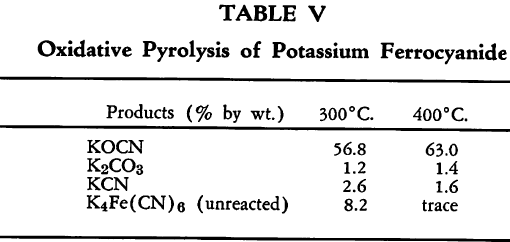
Hydrogen peroxide is said to act exothermically on anhydrous or hydrated potassium ferrocyanide in the absence of water with vigorous gas evolution and formation of free hydroferrocyanic acid and potassium peroxide.
Reaction with Acids
Dry hydrogen chloride is absorbed rapidly by hydrated or anhydrous potassium ferrocyanide. If a crystal of the ferrocyanide is dropped into an ether solution of the same acid in the presence of ferric chloride, the reactivity appears to be greatest at the crystal edges. The deep blue color of ferric ferrocyanide forms there immediately.
Anhydrous potassium ferrocyanide dissolves in hydrofluoric acid with vigorous reaction.
When potassium ferrocyanide is placed in 100 percent sulfuric acid below room temperature, a white coating forms on the crystals. If the mixture is heated to 60 °C., the crystals dissolve, and when the solution is cooled and allowed to stand for some time, a compound suggested to be hydroferrocyanic acid with six moles of sulfuric acid of crystallization precipitates in beautiful plates (See p. 32).
K4Fe(CN)6 + 8 H2SO4 → H4Fe(CN)6.6H2SO4 + 2 K2SO4
As discussed in another section, dilution of the sulfuric acid leads to decomposition of the ferrocyanide ion.
Reaction with Metals
When sodium is fused with potassium ferrocyanide, the major products are said to be potassium cyanide, sodium cyanide, and iron.
K4Fe(CN)6 + 2 Na → 4 KCN + 2 NaCN + Fe
Reaction with Inorganic Salts
When potassium ferrocyanide and sodium thiosulfate are heated together, ferrous sulfide, sodium thiocyanate, sodium sulfate, and sodium sulfide reportedly are found among the products.
If potassium ferrocyanide is fused with potassium carbonate, the mixture melts, darkens, evolves carbon dioxide, and finally becomes almost white. The residue is made up principally of metallic iron, potassium cyanate, and potassium cyanide. If iron oxalate is added to this mixture, reduction to iron powder occurs upon ignition.
The reaction of dry potassium ferrocyanide with ammonium chloride at high temperatures is said to produce ammonium cyanide, potassium chloride, and ferrous cyanide.
K4Fe(CN)6 + 4 NH4Cl → 4 NH4CN + 4 KCl + Fe(CN)2
When mercuric chloride replaces ammonium chloride in the above reaction, the reported products are cyanogen and mercury, which distill, and potassium chloride and ferrous cyanide, which remain as residue.
K4Fe(CN)6 + 2 HgCl2 → 2 (CN)2 + 2 Hg + 4 KCl + Fe(CN)2
When potassium ferrocyanide trihydrate is boiled in vanadium oxytrichloride, it becomes black but does not dissolve. The postulate has been made that the vanadium salt dehydrates the potassium ferrocyanide and is, in turn, partially hydrolyzed to products that form dark-colored addition complexes with the dehydrated ferrocyanide.
Sulfates of potassium and iron have been produced from the pyrolysis of ammonium sulfate with potassium ferrocyanide.
Reaction with Halogens
During some of his early work on fluorine chemistry, Moissan allowed fluorine gas to contact potassium ferrocyanide in the presence of air. Even near room temperature he observed vigorous combustion of the salt with the generation of cyanogen, which burned with a reddish flame.
When heated with excess bromine under pressure at 150-250°C. for about seven hours, potassium ferrocyanide is decomposed with the formation of cyanuric bromide. The reaction gives yields of about 55 percent of theory based on the ferrocyanide, and the identity of the product has been established by hydrolysis to cyanuric acid.
K4Fe(CN)6 + 6 Br2 → 2 C3N3Br3 + FeBr2 + 4 KBr
Reaction with Alkylating Agents
The literature describes a number of reactions of potassium ferrocyanide with such agents as ethyl iodide and dimethyl sulfate to produce esters of hydroferrocyanic acid and their salts. These reactions are described in greater detail in the chapter dealing with hydroferrocyanic acid.
THE FERROCYANIDE ION
Thermal Stability
Neutral Solutions. Aqueous solutions of the alkali metal ferrocyanides are rather stable at room temperature. It has been reported that these solutions slowly decompose, but this has been questioned on the grounds that decomposition would produce toxic cyanide ions. In reality, ferrocyanide solutions are relatively nontoxic. Furthermore, if such decomposition occurred, the odor of hydrogen cyanide would be noticeable. If oxidation of the ferrocyanide ion took place, complex Iron Blues would result because of the simultaneous presence of ferrocyanide, ferricyanide, ferrous, and ferric ions. The formation of these pigments has not been observed.
The ferrocyanide ion decomposes slowly when irradiated at moderate temperatures with an increase in alkalinity, the formation of cyanide ion, and the precipitation of ferrous ferrocyanide or the di(alkali metal) ferrous ferrocyanide. The decomposition is assumed to take place according to the following equations:
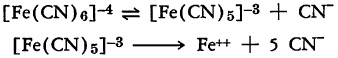
It is accelerated if steam, hydrogen, sulfur dioxide, or carbon dioxide is passed through the boiling solution in order to remove hydrogen cyanide. The reaction continues until the increase in alkalinity causes the evolution of cyanide to cease. At temperatures of 135-350°C. the cyanide liberated is converted to ammonia and formate due to alkaline hydrolysis. This decomposition is less rapid than that observed for the ferricyanide ion and is not complete at temperatures below 100°C.
Alkaline Solutions. Strongly alkaline solutions of the ferrocyanide ion are more stable towards heat than neutral solutions. Decomposition proceeds very slowly to ammonia and the alkali formate, but more actively when ferricyanide is present. No decomposition occurs in the presence of potassium cyanide.
Acid Solutions. Solutions of the ferrocyanide ion, acidified with mineral acids, decompose slowly at room temperature and more rapidly when heated, with the formation of hydrogen cyanide and ferrous ferrocyanide.
3 H4Fe(CN)6 → 12 HCN + Fe2Fe(CN)6
The extent of decomposition appears to depend on the acidity of the solution, increasing as the acidity is increased toward that of hydroferrocyanic acid. The ferroaquapentacyanide ion, [Fe(CN)5H2O]-3, may form as an intermediate. This result has been observed with solutions of hydroferrocyanic acid, and with solutions of alkali metal ferrocyanides after acidification with mineral acids, ammonium chloride, or organic acids. To a large extent, alkali metal, ammonium, or hydrogen ions replace part of the iron in this precipitate.
The ferrocyanide ion in cold solution is relatively stable toward carbonic acid or bicarbonate ion and hydrogen sulfide, but at higher temperatures, even below 100°C., is said to be decomposed by them. When heated with sulfurous acid the complex ion also is destroyed.
It has been mentioned previously that solutions of hydroferrocyanic acid in concentrated sulfuric acid are decomposed by heat with the evolution of carbon monoxide or carbon dioxide. Similar results have been observed for solutions of potassium ferrocyanide in concentrated sulfuric acid. With 98 percent sulfuric acid at 200°C., the evolution of carbon monoxide is slight, but more dilute sulfuric acid causes quantitative liberation of this gas at 180°C.
K4Fe(CN)6 + 8 H2SO4 + 6 H2O → 4 KHSO4 + FeSO4 + 3 (NH4)2SO4 + 6 CO
With further dilution, however, decomposition to cyanide and ferrous ferrocyanide becomes the dominant reaction. Evidence has been presented to indicate that the carbon monoxide results from decomposition of formic acid, which is first formed. Potassium ferrocarbonylpentacyanide, K3Fe(CN)5CO, also has been found in the reaction mixture.
Photochemical Stability
As early as 1795 Berthollet realized that alkaline or neutral ferrocyanide solutions are slowly decomposed by the action of light. This change is slower than that observed for the ferricyanide ion and results in the formation of cyanide and hydroxide ions. The increased alkalinity undoubtedly results from hydrolysis of the cyanide ions as follows:
![]()
Slight increases in pH increase the rate of decomposition, whereas strong alkali is reported to have the opposite effect.
All the evidence presented below supports the presence of a reversible equilibrium of the following nature:
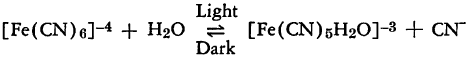
This equilibrium lies entirely to the left in the absence of light. In the first place, the conductivity of neutral or slightly alkaline ferrocyanide solutions does not change upon irradiation, but some difference in potential between an irradiated and an unirradiated one has been observed. This difference is reduced when an irradiated solution is placed in the dark. It also has been noted that solutions of ferrocyanide become yellowish in the presence of light, but the color disappears when the light is removed. Finally, the absorption spectrum of an irradiated ferrocyanide solution indicates the presence of the ferroaquapentacyanide ion.
Although these observations lead to the conclusion that the action of light causes only the dissociation of the ferrocyanide ion in neutral or slightly alkaline solution, several workers have reported that iron hydroxides precipitate as the primary product of irradiation. The formation of ferric hydroxide, however, appears to depend, not upon irradiation, but upon oxidation. None of it is produced in a hydrogen atmosphere, but it precipitates at an increasing rate during irradiation if the concentration of oxygen is increased.
4 [Fe(CN)6]-4 + O2 + 8 OH- + 2 H2O → 4 Fe(OH)3 + 24 CN-
It should be noted that oxidation of alkaline or neutral solutions of ferrocyanides in the absence of light has not been observed.
Irradiated ferrocyanide solutions have an enhanced reactivity toward many reagents. They give deep colors with nitrobenzene, aniline, nitrosodimethylaniline, chloroform guiacum tincture, aminouracil, reduced phenolphthalein, formaldoxime, and sodium azide. These colors have been assumed to result from the presence of the ferroaquapentacyanide ion. It has been noted frequently that hydrogen peroxide is decomposed with the evolution of oxygen by treatment with irradiated, neutral ferrocyanide solutions. This reaction is very slow in the dark, and it has been suggested that the decomposition of peroxide is due to the presence of the ferroaquapentacyanide ion in the irradiated solution.
Contrary to the behavior of neutral or slightly alkaline solutions, acid solutions of ferrocyanide ion are readily oxidized by air to Prussian Blue (p. 35). There is considerable evidence which indicates that irradiation greatly accelerates this oxidation. The effect of various wave lengths on this photoacceleration has been studied, and the oxidation was greatest in blue or green light and smallest in red or yellow light.
Oxidation
The ferrocyanide ion is oxidized to ferricyanide by the action of strong oxidizing agents.
![]()
The various agents that have been used are listed in Table VI. The more interesting ones are discussed briefly below.
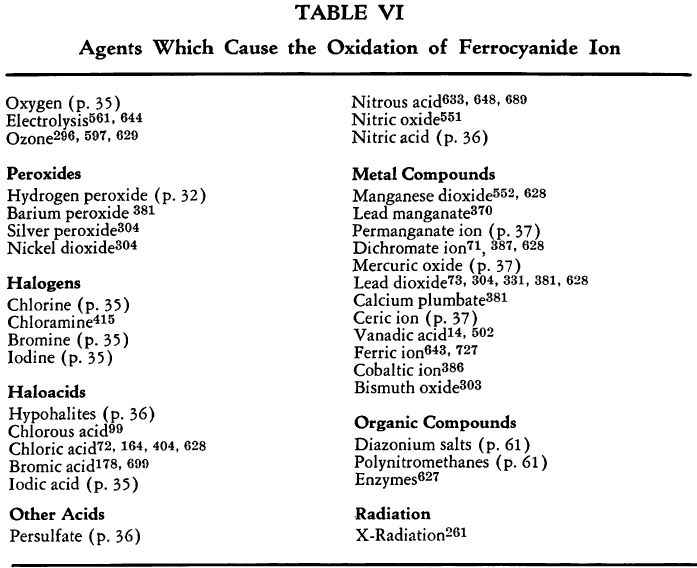
By Atmospheric Oxygen. Neutral or alkaline ferrocyanide solutions are not oxidized by atmospheric oxygen in the absence of light. Acidic solutions are oxidized by air to ferric ferrocyanide and hydrogen cyanide.
7 H4Fe(CN)6 + O2 → Fe4[Fe(CN)6]3 + 2 H2O + 24 HCN
It has been reported that oxidation also occurs if a neutral ferrocyanide solution is shaken with powdered carbon, Fuller’s earth, or silica, due, in this case, to the activity of the adsorbed oxygen. Other, and more effective catalysts are the oxides of elements of atomic numbers 23-29, particularly chromium dioxide, ferric oxide, and cupric oxide.
By Peroxides. The action of hydrogen peroxide on acidified ferrocyanide solutions results in oxidation to ferricyanide. This reaction has been shown to be reversible in the absence of acid, ferricyanide being readily reduced by the hydroxide ion.
![]()
Prussian Blue and ferric oxide also are reported as products from the oxidation of ferrocyanide with hydrogen peroxide.
By Halogens. Gaseous chlorine causes the oxidation of the ferrocyanide ion to ferricyanide. The reaction is quantitative, but it can be reversed if chlorine is removed by silver or if ferrocyanide is precipitated by the addition of a metal ion.
![]()
The ferriaquapentacyanide ion also is reported as a product of the oxidation of the ferrocyanide ion by chlorine.
Oxidation with bromine is similar to that with chlorine. The rate of reduction of bromine by the ferrocyanide ion is equivalent to that by titanium chloride. This reaction is reversible in the presence of phenol.
The oxidation of ferrocyanide with iodine has been the object of much study ever since its discovery in 1839.
![]()
The reaction is known to be reversible, the position of equilibrium being on the left in the presence of acid and on the right in alkaline solutions. Since zinc ferrocyanide is less soluble than zinc ferricyanide, this metal has been used to drive the reaction to the left. In neutral buffered solutions, a considerable excess of iodine is required to effect complete oxidation unless a long period of time is allowed. The reaction proceeds very slowly at room temperature but more rapidly at 40 °C. In strongly acidic solutions, some ferric ferrocyanide is formed. The reverse reaction is strongly accelerated by catalytic amounts of platinum, less so by gold and chromium. This action parallels the polarizability of these metals in ferrocyanide-ferricyanide solutions. If excess iodine is used in the oxidation, it may appear in the complex salt K3Fe(CN)6·KI.
A considerable controversy regarding the actual mechanism of the oxidation has been reported in the literature. Two of the most recent investigations, however, agree that the following reaction is of considerable interest, since nearly all of the iodine present is in the form of the triiodide ion:
![]()
It has been reported that a potassium ferrocyanide solution, treated in the dark with a small amount of iodine, becomes dark red and precipitates Prussian Blue.
By Halogen Oxyacids. Hypochlorous acid rapidly converts the ferrocyanide ion to nitroprusside, [Fe(CN)5NO] =, at 33-34°C. At 70-80°C. the products are said to be ferricyanide, nitroprusside, and ferric ions. Reaction with alkaline hypobromite solution at room temperature produces the ferricyanide ion very slowly if at all. If the solution is boiled, nitrogen is evolved and a red, ferric-containing precipitate is formed.
Rate studies of the oxidation of the ferrocyanide ion by iodate in the presence of hydrochloric acid show that the reaction is similar to the Landolt oxidation of sulfite ion.
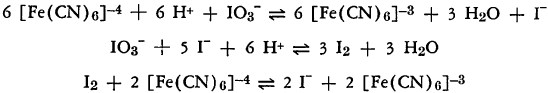
The color changes observed are a preliminary yellowing due to the ferricyanide ion, followed by the iodine coloration. Since the last reaction is reversible, oxidation to ferricyanide is not complete in the absence of excess iodate.
By Persulfates. Neutral solutions of ferrocyanides are slowly oxidized by the persulfate ion at ordinary temperatures. The reaction at 60 °C. is quite rapid.
![]()
In hot, acid solutions, the ferricyanide is destroyed, part of the cyanide being lost as HCN and part oxidized to cyanogen gas. The oxidation is accelerated by the presence of potassium nitrate. Kinetic studies indicate that the reaction is bimolecular. It has been reported that small amounts of ferrocyanide first decompose but then stabilize solutions of persulfate. The stabilization apparently is due to the ferricyanide ion.
By Nitric Acid. When nitric acid is added to an aqueous ferrocyanide solution, hydroferrocyanic acid is precipitated and then decomposed, forming ferricyanide, nitroprusside, hydrogen cyanide, cyanogen, carbon dioxide, oxamide, and nitrous acid. When potassium ferrocyanide is dissolved in concentrated nitric acid, heat is evolved and Prussian Blue is formed. When heated further, the solution becomes viscous with the generation of nitrogen, cyanogen, carbon dioxide, and nitrogen oxides and then again fluid with precipitation of ferric nitrate and potassium nitrate. Aqua regia and concentrated sulfuric acid containing a few drops of nitric acid similarly convert all of the iron to a ferric salt, the sulfuric-nitric acid mixture acting quantitatively.
By Permanganate Ion. The ferrocyanide ion is quantitatively oxidized to ferricyanide in acid solution by the action of the permanganate ion.
![]()
With excess permanganate the ferricyanide ion is decomposed slowly to carbon dioxide, ferric oxide, and nitrogen oxides. This decomposition is quantitative in the presence of bromide. If the oxidation is conducted with too high a pH or in too concentrated a solution, a precipitate of K2MnFe(CN)6 is formed. At high concentrations of acid the reaction is not quantitative because of the formation of reducing by-products by the decomposition of ferricyanide. Hydrogen cyanide also is generated under highly acidic conditions.
In alkaline solutions the end products from the oxidation of ferrocyanide with permanganate ions are said to be manganite and ferric oxide, although the up-take of permanganate is thought to be no greater than in neutral solution. Other observations on this reaction suggest that the true end point precedes the permanganate coloration and that it is immaterial to the success of the titration whether hydrochloric or sulfuric acid is employed. Several workers have reported studies on the reaction of permanganate ion with various metal ferrocyanides such as lead, zinc, cadmium, silver, manganous, cobaltous, nickel, and cupric.
By Mercuric Oxide. Mercuric oxide reacts with boiling ferrocyanide solutions with the formation of ferric oxide and mercury cyanides. If the hot solution is acidified, ferrous ferrocyanide, which may be oxidized by the mercuric oxide with the precipitation of metallic mercury, is formed.
By Ceric Salts. One of the most accurate methods for determining ferrocyanide is by titration with the ceric ion.
[Fe(CN)6]-4 + Ce+4 → [Fe(CN)6]-3 + Ce+3
The method is more exact than titration with permanganate. The end point may be determined potentiometrically or by the color produced by the presence of the ferric ion.
By Organic Compounds. Tetranitromethane is reduced quantitatively by ferrocyanide solutions.
2 [Fe(CN)6]-4 + C(NO2)4 → 2 [Fe(CN)6]-3 + C(NO2)3- + NO2-
A similar reaction occurs with bromotrinitromethane, but not with trichloro- or tribromonitromethane.
Miscellaneous. Potassium ferrocyanide is said to inhibit temporarily the oxidation of acrolein and to retard the oxidation of the ferrous ion by oxygen. On the other hand, the ferrocyanide ion induces the oxidation of tartaric acid by chromate.
Reaction with Cations
Ferrocyanides have been prepared from nearly every metallic element in the periodic system. In general these are prepared by the addition of aqueous potassium ferrocyanide to a weakly acidic solution of the metal salt, with the usually insoluble metal ferrocyanides separating. In most cases, the precipitates are contaminated by constant or variable amounts of potassium ferrocyanide held by adsorption, solid solution, or double salt formation. In difficult cases, the potassium-free metal ferrocyanide can be obtained most conveniently by using aqueous hydroferrocyanic acid as the precipitant.
The preparation and some of the properties of most of the known, simple, metallic ferrocyanides are outlined in Table VII. The symbols used in the table are explained on p. 42.
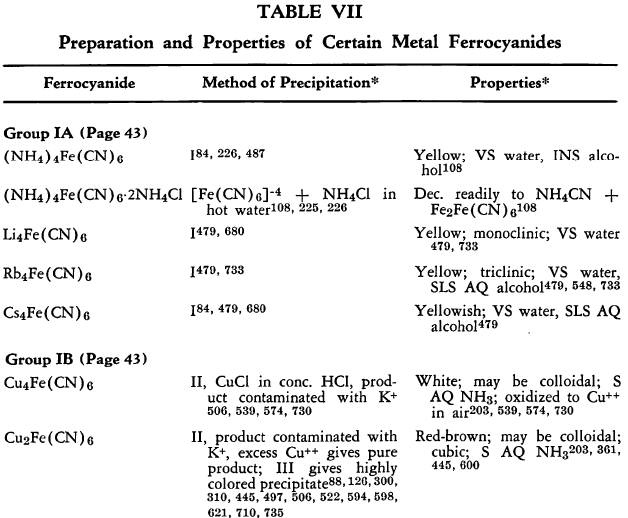
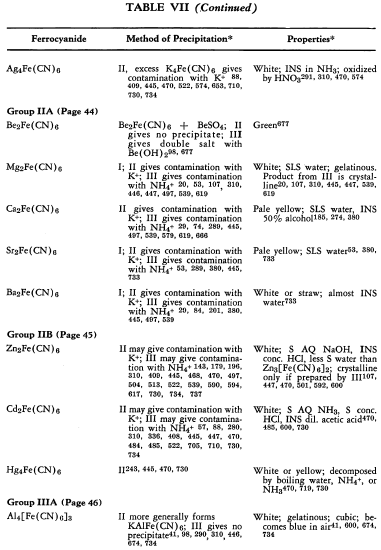
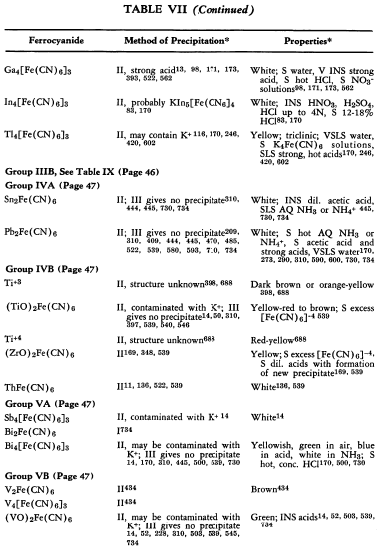
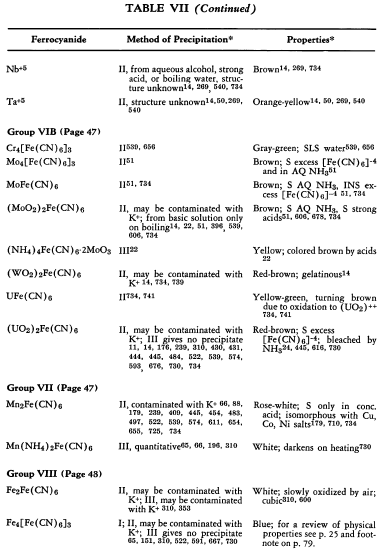
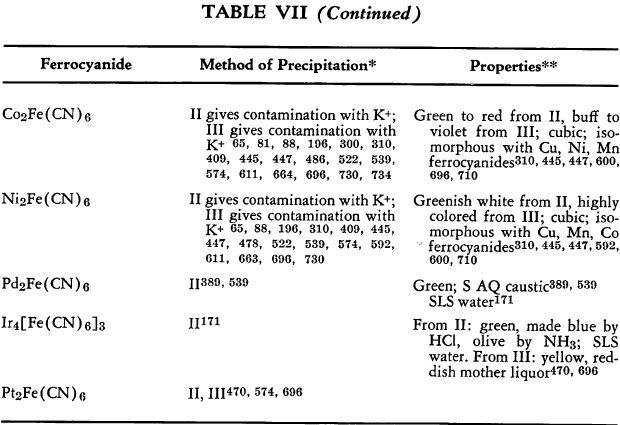
The relative solubilities of the metallic ferrocyanides are given in Table VIII. In the following sections information which is supplementary to that appearing in the tables is discussed briefly. The elements appear in their classical groups.
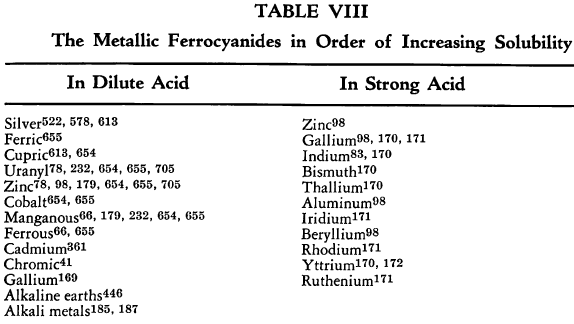
Other data regarding the relative solubilities of the various metal ferrocyanides have been obtained by precipitation of these ferrocyanides in the presence of the permanganate ion. Tests then were made on the extent of precipitation that took place before oxidation to ferricyanide was complete with the following results:
- Cadmium No precipitation
- Cobalt Complete precipitation
- Lead Complete precipitation
- Manganous Complete precipitation
- Nickel Complete precipitation
- Silver Complete precipitation
- Zinc Complete precipitation
Group IA, Alkali Metals—Lithium, Sodium, Potassium, Rubidium, Cesium, Ammonium. As a class, the alkali metal ferrocyanides are soluble in water, but may be precipitated by the addition of ethanol to their aqueous solutions. The lithium salt is the most soluble in water-alcohol mixtures and the cesium salt is the least soluble, the other metals falling in order between these limits. A similar solubility relationship is found for the alkali metal calcium ferrocyanides. Lithium and sodium ferrocyanides are not precipitated by the calcium ion even from aqueous alcohol, but the potassium salt is; and rubidium and cesium calcium ferrocyanides are precipitated quantitatively from water by calcium salts. Double ferrocyanides of lithium and other alkali metals have been prepared.
Certain ammonium ferrocyanides, notably 2NH4Cl·(NH4)4Fe(CN)6 and (NH4)HFeFe(CN)6, are formed by the decomposition of hot ferrocyanide solutions in the presence of ammonium chloride. In the presence of other acids, potassium ferrocyanide is decomposed with the formation of K2FeFe(CN)6 (see p. 32).
A great number of double ferrocyanides of potassium with other metal cations have been prepared from potassium ferrocyanide solutions. These will be discussed below in describing the other metallic ferrocyanides.
Group IB—Copper, Silver, Gold. Metallic copper and silver are very slightly soluble in cold ferrocyanide solutions. Silver dissolves if the solution is boiling, because the ferrocyanide decomposes to alkali cyanide. Gold powder or foil dissolves forming the complex auricyanide ion, [Au(CN)4]-.
As with most metallic ferrocyanides that are precipitated from potassium ferrocyanide solutions, those of the Group IB metals frequently form potassium-contaminated precipitates. In this group, the copper ferrocyanides are most liable to contamination, and excess cupric ion or high acidity is required in order to obtain the simple ferrocyanide. Various methods of examining these contaminated precipitates have resulted in a variety of suggestions regarding the structure of the products formed:
Polarimetry K2Cu3[Fe(CN)6]2 by addition of Cu++ to K4Fe(CN)6 solution
Conductimetry 3Cu2Fe(CN)6·K4Fe(CN)6 by addition of K4Fe(CN)6 solution to Cu2Fe(CN)6
Amperometry Absorption of K4Fe(CN)6 on Cu2Fe(CN)6 up to limit represented by 5Cu2Fe(CN)6·K4Fe(CN)6
X-Ray Diffraction Absorption or dissolution of K4Fe(CN)6 by Cu2Fe(CN)6
X-Ray, Electron Ray Solid solutions of [CuCuFe(CN)6]m·[K2CuFe(CN)6]n where m and n vary continuously, by addition of K4Fe(CN)6 solution to Cu++
This last case is of interest because of the formation of what appears to be a stable cupriferrocyanide ion, [CuFe(CN)6]=. Evidence for the presence of the corresponding acid, H2CuFe(CN)6, in solutions prepared from copper sulfate and excess hydroferrocyanic acid also has been found. A variety of salts of this acid have been prepared as well as other mixed cupric ferrocyanides, MCuFe(CN)6. Gray-blue ferrocyanides are formed by dipyridyl- cupric and phenanthroline-cupric ions.
Studies dealing with silver ferrocyanide show that the addition of excess potassium ferrocyanide to silver solutions results in the formation of contaminated precipitates.
Conductimetry, KAg3Fe (CN)6
Conductimetry 3Ag4Fe(CN)6·K4Fe(CN)6
Potentiometry Contamination of Ag4Fe(CN)6
X-Ray Diffraction Double salt
Silver ferrocyanide is extremely insoluble in water, its solubility being intermediate to that of silver chloride and silver bromide. Thus, silver chloride dissolves in solutions of potassium ferrocyanide with precipitation of silver ferrocyanide, whereas silver bromide and iodide do not. The ferrocyanide also is less soluble than silver chromate, precipitates of the latter being converted into silver ferrocyanide by exposure to the ferrocyanide ion. A solution clouded by silver cyanide is cleared by a drop of potassium ferrocyanide solution.
Alkaline and ammoniacal solutions of ferrocyanide are decomposed by the addition of silver salts, possibly because of the removal of the cyanide ion by the silver.
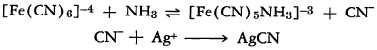
One of the final products is believed to be ferrous cyanide. Similarly, silver ion causes the decomposition of boiling ferrocyanide solutions.
[Fe(CN)6]-4 + 6 Ag+ → 6 AgCN + Fe++
Auric ferrocyanide is not formed by the addition of a gold salt to a ferrocyanide solution. On boiling, however, a green color forms, and if ammonia is added, a precipitate is obtained. In the presence of potassium carbonate, ferrocyanide and auric ions produce soluble blue complex cyanides. Precipitation of mixtures of gold cyanides and Prussian Blue, Fe4[Fe(CN)6]3, is said to occur in hydrochloric acid solutions. Both gold cyanide and gold hydroxide slowly dissolve in ferrocyanide solutions with the formation of complex cyanides and the precipitation of ferric oxide.
Group IIA, Alkaline Earths—Beryllium, Magnesium, Calcium, Strontium, Barium, Radium. The chief characteristic of the alkaline earth ferrocyanides is the ease with which double salts are prepared with the alkali metal ferrocyanides. It is not always clear from the literature exactly what are the structures of the precipitates obtained from concentrated aqueous solutions. However, it seems certain that from aqueous solutions containing alkali metal, alkaline earth, and the ferrocyanide ion, one usually isolates the double salt, e.g., CaK2Fe(CN)6. Although these double salts, and the normal alkaline earth salts as well, are partially soluble in water, as a general rule the ammonium-containing alkaline earth ferrocyanides are much less soluble.
Alkaline earth ferrous ferrocyanides, e.g., CaFeFe(CN)6, may be prepared by boiling aqueous hydroferrocyanic acid solutions with an alkaline earth sulfate or chloride. The literature reports that this reaction takes place with magnesium sulfate but not with magnesium chloride. Solutions of alkaline earth ferrocyanides are decomposed slowly by carbon dioxide in the presence of air with the formation of the metal carbonate and hydroferrocyanic acid, together with small amounts of hydrocyanic acid and ferric ferricyanide.
It has been reported that calcium does not form double salts with most heavy metal ferrocyanides, although such salts with thallium and copper are reported. Interesting and highly-colored, insoluble ferrocyanides are obtained when alkaline earth or alkali metal-alkaline earth ferrocyanides are treated with heavy metal ions such as Cu++, Sn++, Sb+++, Bi+++, Co++, or Ni++.
Group IIB—Zinc, Cadmium, Mercury. Zinc and cadmium ions behave similarly toward solutions of potassium ferrocyanide, forming white precipitates of the metal salt from neutral, acidic, or ammoniacal solutions. If the ferrocyanide is added gradually to an acidic solution of zinc or cadmium salt, the simple metal ferrocyanide, M2Fe(CN)6, is first precipitated. Before the equivalence point is reached, however, a mixed salt begins to form. The reported investigations do not agree exactly as to the constitution of the mixed salt formed. Conductimetric, polarimetric, and potentiometric data suggest the composition K2Zn3[Fe(CN)6]2 in the case of zinc; but more recent conductimetric studies lead to different constitution, 3Zn2Fe(CN)6. K4Fe(CN)6. Thermometric studies for the case of cadmium are interpreted as evidence for a mixed salt having the structure K8Cd10[Fe(CN)6]7, whereas conductivity measurements suggest K2Cd3[Fe(CN)6]2 and 5Cd2Fe(CN)6·4K4Fe(CN)6. Some of these differences in opinion may be due to different reaction conditions; the proportions of reactants used and the pH of the solution are known to be important factors. In solutions that are 1-2 Normal in mineral acid, only the mixed salt is formed, but from acetic acid of equivalent concentration only the simple salt results. Similarly, the product from ammoniacal solutions is simply cadmium ferrocyanide.
The double ferrocyanides of zinc with potassium and ammonium ions are less soluble than the simple zinc ferrocyanide. This may account for the observation that only a mixed salt is formed by the addition of a zinc salt to potassium ferrocyanide solutions at all concentrations of acid. Since a consistent product, probably 3Zn2Fe(CN)6·K4Fe(CN)6, is formed, this manner of addition is preferred for quantitative analyses. Double salts of zinc ferrocyanide with various alkali and alkaline earth metals and with copper are reported.
The reaction of mercuric ion with potassium ferrocyanide in neutral, acid, or ammoniacal solution leads to decomposition rather than to the formation of a stable salt. The visual observation is the formation of a white precipitate of ferrous ferrocyanide or dipotassium ferrous ferrocyanide. The precipitate slowly turns blue or gray, owing to oxidation to Prussian Blue by the mercuric ion, and may contain mercurous compounds and metallic mercury. In the solution from which precipitation occurs, various salts and ions have been observed, including mercuric cyanide, the mercurous ion, metallic mercury, the ferric ion, and the ferrous ion.
Addition compounds are formed between mercuric cyanide and potassium ferrocyanide in which three molecules of mercuric cyanide are involved. Mercuric iodide dissolves in ferrocyanide solutions forming K4Fe(CN)6·HgI·2H2O, which crystallizes when the solution is cooled.
Both metallic mercury and mercuric oxide decompose strongly acidic ferrocyanide solutions with the formation of a “mercury-amide” compound.
Group IIIA—Boron, Aluminum, Gallium, Indium, Thallium. Ferrocyanides have been prepared from aluminum, gallium, indium, and thallium ions. The aluminum salt is said not to be precipitated in ammoniacal or concentrated hydrochloric acid solutions. For many years it was believed that no precipitation occurred from neutral or slightly acidic solutions either. Oblong standing, however, a gelatinous aluminum ferrocyanide is precipitated.
Gallium forms a simple ferrocyanide from neutral or acid solutions. If the precipitate is formed at temperatures above 70 °C., it is blue in color and partly decomposes. It is soluble in water but not in the strongly acid mother liquid.
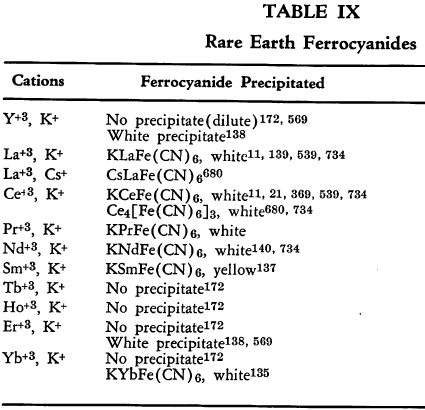
Group IIIB—The Rare Earths. The various rare earth ferrocyanides are listed in Table IX.
Group IV—Germanium, Tin, Lead, Titanium, Zirconium, Hafnium, Thorium. The metals of Group IV (see Table VII), except for titanium, show very little tendency to form mixed ferrocyanides in the presence of alkali metals. The ferrocyanides formed are quite insoluble in water, but generally dissolve in ammoniacal or strongly acid solutions.
Group V—Arsenic, Antimony, Bismuth, Vanadium, Niobium, Tantalum. Not a great deal is known regarding the ferrocyanides of the elements of the fifth group of the Periodic System. Of the A group elements, only the bismuth ferrocyanides have been studied at any length. Insoluble ferrocyanides of arsenic are unknown. Various precipitates of undetermined compositions are formed from the salts of the transition elements, vanadium, niobium, and tantalum.
Group VI—Tellurium, Chromium, Molybdenum, Tungsten, Uranium. Of the elements in Group VIA only tellurium is reported in the ferrocyanide literature. Tellurium tetrachloride is converted into telluric acid by treatment with ferrocyanide ion. After several hours, Prussian Blue is precipitated.
Interesting ferrocyanides are formed by the elements of Group VIB (see Table VII). Besides a normal chromic ferrocyanide, chromium forms several other more complex salts. Those prepared from the amminochromic complexes have been well investigated. Ferrocyanides have been prepared from trivalent and tetravalent molybdenum and from the molybdates. The latter form red-brown (MoO2)2Fe(CN)6 in acid solutions. In the presence of ammonium ion the following equilibrium apparently exists between this product and the yellow ammonium ferrocyanidemolybdic oxide compound:
![]()
A similar equilibrium may exist for uranyl ferrocyanide, (UO2)2Fe(CN)6, which also is bleached by ammonia or ammonium carbonate.
The molybdates, tungstates, and uranates do not react easily with ferrocyanide in neutral or alkaline solutions. The products from acid solutions are contaminated with potassium ferrocyanide, particularly if the latter is present in excess. With the uranates, the simple ferrocyanide is formed only in the presence of acetic acid. Under other conditions the product is the mixed salt 5 (UO2) 2Fe(CN)6·3K4Fe(CN) 6.
Group VII—Manganese, Masurium, Rhenium. Of the metals in Group VII, only the ferrocyanides of manganese have been studied. Addition of potassium ferrocyanide to aqueous manganous salt solutions causes precipitation of a complex double salt, 5Mn2Fe(CN)6·4K4Fe(CN)6. Variations in this composition may be caused by changes in temperature or acidity. From ammoniacal solutions the product which precipitates quantitatively is believed to be Mn(NH4)2Fe(CN)6.
Group VIII—Iron, Cobalt, Nickel, Ruthenium, Rhodium, Paladium, Osmium, Iridium, Platinum. The formation of iron ferrocyanides has been studied exhaustively for two centuries because of their valuable pigment applications. The extent and complexity of this work make it beyond the scope of this book. In general, however, the addition of potassium ferrocyanide to ferric solutions at first causes the formation of the simple ferrocyanide, Fe4[Fe(CN)6]3, often called “insoluble” Prussian Blue. Additional potassium ferrocyanide leads to complex salts of variable compositions, “soluble” Prussian Blue, Milori Blue, and many others. (A brief discussion of the structure of some of these appears on p. 26). A blue precipitate also is formed on treating potassium ferric oxalate with ferrocyanide. In this case the precipitate gradually becomes white on exposure to light.
Ferrous ferrocyanide readily adsorbs alkali metal ferrocyanides and so is not obtained easily as the simple salt. It may be formed, however, by addition of ferrous salts to hydroferrocyanic acid, provided that oxygen and air are excluded. It also results from the thermal decomposition of acidified ferrocyanide solution as discussed elsewhere (p. 32). Various alkali metal ferrous ferrocyanides may be prepared in this manner by the inclusion of an alkali metal salt in the acidified ferrocyanide solution. A ferrocyanide of the nitrosoferric ion has been prepared.
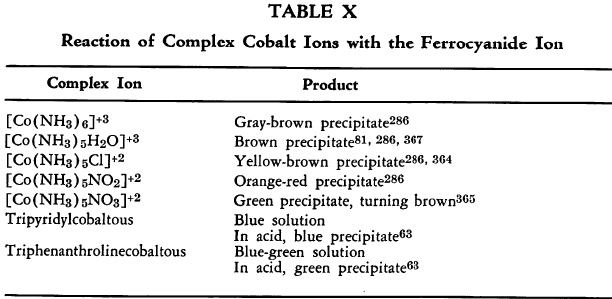
Bivalent cobalt and nickel ferrocyanides (Table VII) also tend to co-precipitate potassium ions. The result is a variation in color that is particularly marked when precipitation is effected from ammoniacal solutions. Various structures of the types, M2Fe(CN)6·xK4Fe(CN)6, have been proposed. The composition of these salts is known to vary with the order of addition of the two reagents as well as with other reaction conditions. Certain complex cobalt ferrocyanides are listed in Table X.
Only meager information exists regarding the ferrocyanides of the other elements of Group VIII. These data are assembled in Table VII. Ferrocyanides of aquopentamminerhodium ion and hexammineiridium ion also have been prepared.
Reaction with Anions
Most of the reactions of ferrocyanide ions with other anions involve oxidation, which is discussed elsewhere (pp. 34-38). The sulfide and nitrite ions are interesting in other ways, however, and these are discussed below.
Sulfide. The sulfide ion causes a very slow decomposition of ferrocyanide solutions in the presence of light. A slight precipitation of ferrous sulfide is observed. In hot, ammoniacal ferrocyanide solutions sodium sulfide causes some formation of thiocyanate ion. This is not observed in the absence of ammonia. The action of hydrogen sulfide on ferrocyanide solutions is noted elsewhere (p. 32).
Nitrite. The nitrite ion causes the formation of a bright yellow coloration in ferrocyanide solutions in the presence of acetic acid. This is due to the formation of the ferricyanide ion and nitric oxide.
![]()
Nitroprusside, [Fe(CN)5NO]-2, is formed from ferrocyanide and acidic nitrite or nitrate solutions on boiling. In the cold, the reaction goes slowly and incompletely owing to the formation of by-products such as ferric oxide, ferrous ferrocyanides, and the nitritoprusside ion.
Reaction with Nonmetals
Oxygen. Reactions of the ferrocyanide ion with oxygen and ozone are discussed elsewhere (p. 35).
Hydrogen. The ferrocyanide ion may be hydrogenated in acid solutions in the presence of palladium to monomethylamine. With nascent hydrogen (from aluminum) only small amounts of ammonia and methylamine are obtained.
Nitric Oxide. Acidic ferrocyanide solutions combine with nitric oxide forming unknown products.
Carbon Monoxide. Carbon monoxide reacts with ferrocyanide solutions in the cold so slowly as to be negligible. Under the influence of heat, however, the reaction is much more rapid with the formation of the ferrocarbonyl- pentacyanide ion.
[Fe(CN)6]-4 + CO + 2 H20 → [Fe(CN)5CO]-3 + HCO3- + NH3
The reaction is not noticeably reversible. The formation of the ferrocarbonyl- pentacyanide ion also has been observed during the decomposition of ferrocyanide ion with hot, concentrated sulfuric acid. In this reaction, carbon monoxide is known to be formed from the decomposition (see p. 32). The complex ferrocarbonylpentacyanide ion is not formed, however, in hot, aqueous ferrocyanide solutions in the absence of carbon monoxide or mineral acid.
Miscellaneous Nonmetals. At elevated temperatures sulfur reacts with aqueous solutions of ferrocyanide ions to produce thiocyanates.
[Fe(CN)6]-4 + 6 S → 4 CNS- + Fe(CNS)2
The ferrocyanide ion does not react with arsine. When heated with hydroxylamine, the ferrocyanide ion is converted into nitroprusside and ammonium violet. Both alkaline and acid solutions of hydrazine cause the decomposition of ferrocyanide. It is said that when vapors of carbon disulfide, sulfur dioxide, or diethyl ether are blown with air over paper saturated with ferrocyanide solution, a green coloration results. The air, of course, may be responsible for this behavior.
Reaction with Organic Compounds
Under this heading are discussed only the reactions with organic compounds which involve the ferrocyanide ion. Reactions with nonaqueous ferrocyanides are to be found elsewhere (pages 31 and 64).
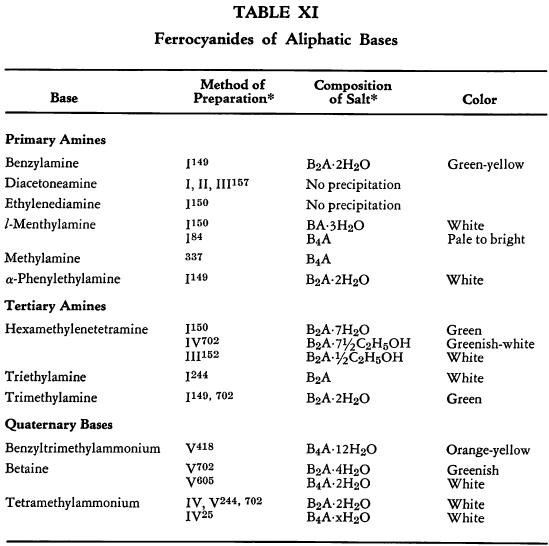
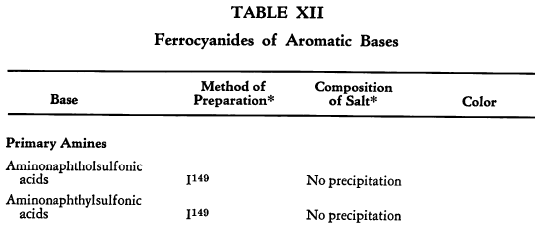
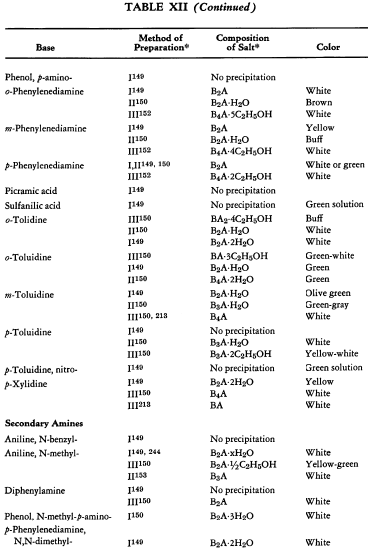
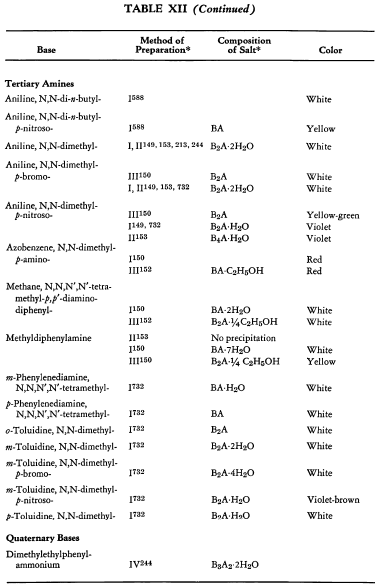
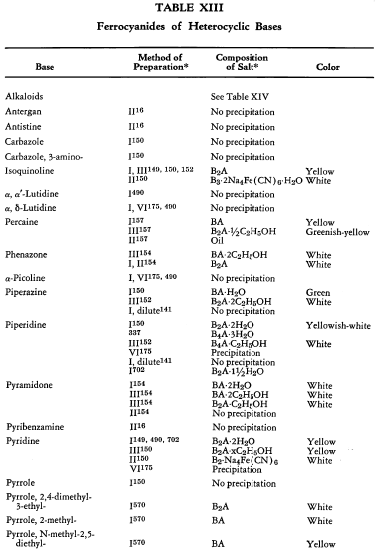
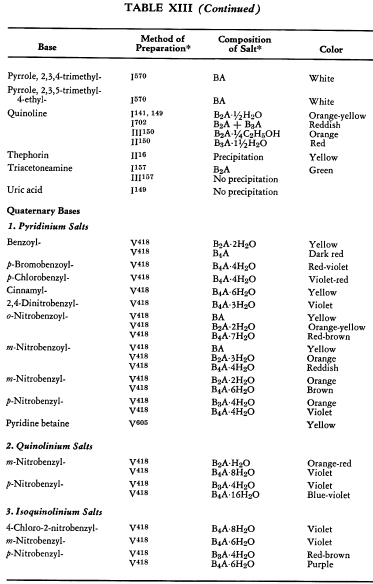
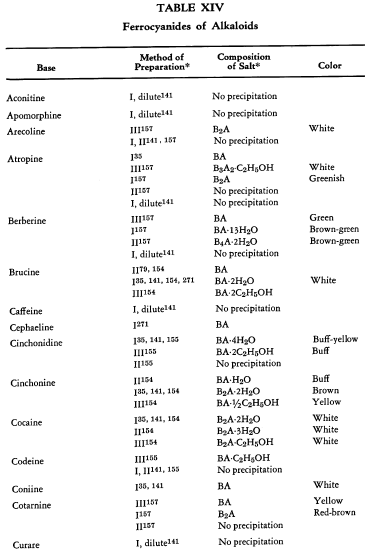
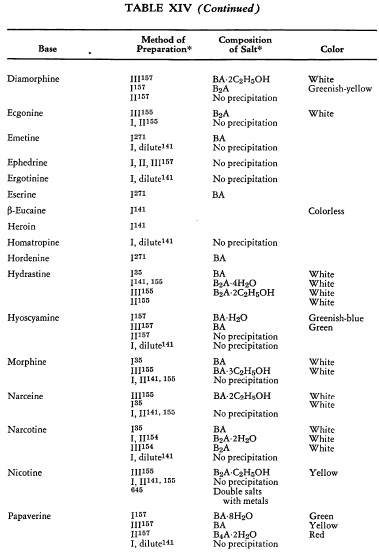
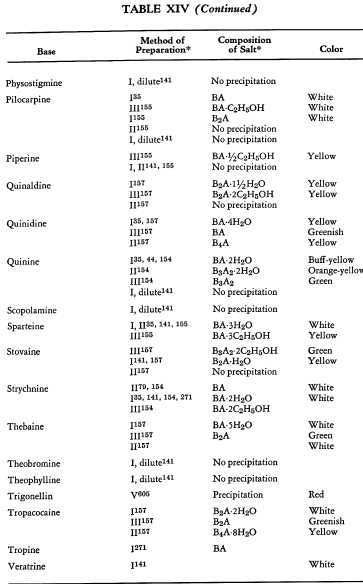
Salt Formation. Ferrocyanides of a large variety of organic bases have been prepared. These have been tabulated for convenient reference in five tables, each containing bases that fall into the following categories: Table XI— aliphatic amines; Table XII—aromatic amines; Table XIII—heterocyclic amines; Table XIV—alkaloids; Table XV—miscellaneous amines.
Six general preparative methods have been used, and each is designated in the tables by its number.
- I. A saturated, aqueous alkali-metal ferrocyanide solution is added to a highly acidified solution of the base in water.
- II. A saturated, aqueous alkali-metal ferrocyanide solution is added to a neutralized solution of the base in water.
- III. An alcoholic solution of hydroferrocyanic acid is added to the free base in alcohol.
- IV. An aqueous solution of hydroferrocyanic acid is added to a solution of the free base in water.
- V. A saturated, aqueous alkali-metal ferrocyanide solution is added to a quaternary salt of the base in water.
- VI. Aqueous potassium ferrocyanide is added to a solution of the base in water.
The organic ferrocyanides have various compositions depending upon the method used for their preparation. These compositions generally cover the range BA, B3A2, B2A, B3A, B4A, where B is used to represent the cation and A the anion portion of the molecule. Thus B4A may be (RNH2)4·H4Fe(CN)6 or (R4N)4Fe(CN)6. Water or alcohol of crystallization is encountered in the great majority of cases. In Tables XI-XV the above designations are used to indicate the types of salts prepared.

In general, the organic ferrocyanides are colored, crystalline compounds. They are usually infusible and insoluble or slightly soluble in cold water. Their solutions vary in pH and are relatively stable at room temperature. At their boiling points, however, aqueous solutions of these salts decompose in different ways, depending upon pH, the presence of light or air, and other factors that have been described more completely in a previous section (p. 31). Salts of the tertiary bases are the most highly colored and the most stable. When the solid salts are heated, they often change color, progressing through green and blue to black with retention of crystal structure. Salts of the aliphatic bases are more soluble than the aromatics and increase in solubility in the order: tertiary<secondary<primary. In the aromatic series, e.g., the methyl- anilines, this order is reversed. Ferrocyanides of diamino compounds tend to be relatively unstable and more insoluble.
Various schemes for the separation of mixtures of organic bases by taking advantage of different solubilities of the ferrocyanide salts have been developed. These include the separation of:
- Diphenylamine from aniline
- Pyridine from its homologs
- Quinoline from pyridine
- Quinoline from isoquinoline
- o-Toluidine from p-toluidine
- Mixtures of alkaloids
Several reactions of the ferrocyanide ion with metal-organic complexes have been reported. These reactions are presumed to involve salt formation of some type. Thus, solutions of the dipyridyl-cupric ion give a gray-violet coloration with the ferrocyanide ion, while those of the phenanthroline-cupric ion cause a gray-blue precipitate to form. Blue colors also have been observed with the tridipyridyl-cobaltous ion and with the triphenanthroline-cobaltous ion. Yellowish crystals are formed by the action of ferrocyanide on tripyridyl-ferrous, tripyridyl-nickelous, and triphenanthroline-nickelous solutions.
Tertiary phosphine oxides form salts in aqueous or alcoholic solutions of hydroferrocyanic acid. Most of these salts occur as small, colorless, alcohol- insoluble crystals that turn green in damp air. The phosphine oxides reported are the trimethyl, triethyl, tripropyl, and triphenyl compounds.
2 R3PO + H4Fe(CN)6 → 2 R3PO·H4Fe(CN)6
Acidified ferrocyanide solutions precipitate sulfonium ferrocyanides from solutions of sulfonium salts. Thus, triethylsulfonium trihydroferrocyanide results from the reaction of an acidified ferrocyanide solution with triethylsulfonium bromide.
(C2H5)3S+Br- + H4Fe(CN)6 → (C2H5)3SH3Fe(CN)6 + HBr
Other sulfonium salts so prepared are the diethylbenzyl, diethylbutyl, dibenzylbutyl, tribenzyl, and dibenzylbenzoyl.
Ammonium ferrocyanide is formed by the reaction of acetamide with acidic ferrocyanide solutions.
Oxidation and Reduction
The ferrocyanide ion is oxidized quantitatively by tetranitromethane and by bromotrinitromethane (see p. 37).
Oxidation of the ferrocyanide ion by aromatic diazonium salts is believed to produce the ferricyanide ion and a free aryl radical.
C6H5N2+ + [Fe(CN)6]-4 → C6H5 + N2 + [Fe(CN)6]-3
The radicals so formed react with the diazonium salts to form arylazodiaryls as the chief products.
![]()
The reaction proceeds most smoothly in the presence of a sodium acetate buffer at pH 4-5. Yields of about 20 percent are obtained with aniline, toluidines, and chloroanilines. With anisidines and nitranilines the yields are less than one percent of the theoretical. From the p-chloroaniline reaction a residue containing 4,4′-dichloroazobenzene and p-chlorobenzonitrile has been obtained.
Boiling aqueous potassium ferrocyanide solution causes the conversion of chloral hydrate to potassium dichloroacetate.
3 Cl3CCHO·H2O + 2 K4Fe(CN)6 → 3 Cl2CHCO2K + 3 KCl + 2 Fe(CN)2 + 2 KCN + 6 HCN
By a similar sort of reaction, trichlorobutyraldehyde is converted into monochlorocrotonic acid.
The pyrimidine ring of 6-methyluracil is said to be ruptured by oxidation caused by treatment with an irradiated, aerated ferrocyanide solution. Alkaline ferrocyanide has been reported to effect the oxidative ring closure of N-thioacetyl-m-anisidine to 5- and 7-methoxy-2-methylbenzothiazole.
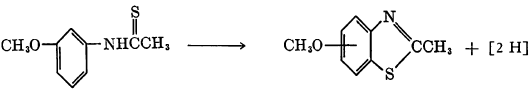
Reaction with Aldehydes and Ketones. If a potassium ferrocyanide solution is refluxed with formaldehyde, the products formed are potassium glycolate, dipotassium ferrous ferrocyanide, and ammonia. The observed products probably result from a premliminary decomposition of the ferrocyanide (p. 31).
2 K4Fe(CN)6 + 6 H2O → K2FeFe(CN)6 + 6 HCN + 6 KOH
CH2O + HCN + KOH + H2O → HOCH2COOK + NH3
Insoluble ferrous and ferric salts also have been observed in this reaction and the solution becomes strongly alkaline.
With many aldehydes and ketones, acidified ferrocyanide solutions form colored crystalline or semicrystalline adducts of unknown structure. (See Hydroferrocyanic Acid p. 65.) Adducts have been obtained from paraformaldehyde, aromatic aldehydes, aliphatic and cyclic ketones. Addition compounds of ferrocyanides with hexamethylenetetramine also have been observed.
Reaction with Proteins and Glucosides
A great many proteins are precipitated by acidic solutions of the ferrocyanide ion. Wool and silk combine with ferrocyanide at pH values of less than 5.0 and less than 4.0, respectively. Interactions with gelatin and casein are reported. The activity of pancreatic proteinase for the hydrolysis of caseinogen is said to be stimulated by ferrocyanide or ferricyanide ions. Colorations result from the reaction of the ferrocyanide ion with digitoxin and with hematoxylin.
Reaction with Miscellaneous Organic Compounds. Adducts of ferrocyanides with ethyl oxalate and with ethylene oxide have been reported, but their structure is unknown. The influence of hydrazine and its salts, hydroxylamine, carbon disulfide, and diethyl ether, on the ferrocyanide ion have been noted in a previous section (p. 50).
Hydroferrocyanic Acid

Ferrocyanides Usage & Applications
Ferrocyanide Methods of Analysis
Ferrocyanide Toxicity